Women’s Health Beliefs and Intention to Use Chemoprevention for Breast Cancer
Objectives: To investigate the associations between women’s health beliefs and their intention to use chemoprevention.
Sample & Setting: Participants were postmenopausal women (N = 400) aged 50–64 years who were recruited for a study on mammographic breast density.
Methods & Variables: Participants completed a screening mammogram and breast cancer health belief questionnaires. The authors regressed intention to use chemoprevention onto health belief scores (breast cancer fatalism, fear, perceived threat, perceived benefits, barriers, and self-efficacy).
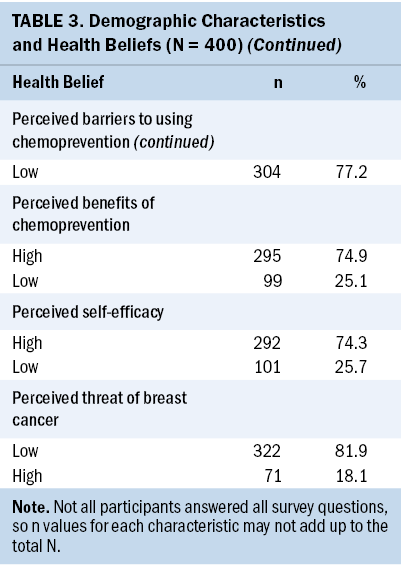
Results: Nearly half of the participants indicated that they would be interested in using chemoprevention if they were found to be at high risk for developing breast cancer. Women who reported higher perceived benefits of chemoprevention, higher perceptions of their ability to use chemoprevention (self-efficacy), and fewer logistic barriers to seeking health care had significantly higher intention to use chemoprevention.
Implications for Nursing: Interventions aimed at reducing logistic barriers to health care may increase the uptake of chemoprevention among at-risk women. In addition, women at the time of mammography and women with higher levels of education may be motivated to consider using chemoprevention.
Jump to a section
Breast cancer presents a major health challenge worldwide. It is the most diagnosed cancer in women. Of all new cancer cases, 25% are breast cancer, and an estimated one out of every eight women (12.4%) will be diagnosed with breast cancer during their life span (Howlader et al., 2020). In addition, the incidence of breast cancer has been steadily increasing since 2005 (American Cancer Society, 2023). Although advances in treatment and early detection undoubtedly contribute to the long-term survival of people with breast cancer, the key to decreasing the incidence of breast cancer is primary prevention (Thorat & Balasubramanian, 2020).
Researchers have estimated that 15% of women in the United States are eligible to use chemoprevention because risk factors place them at increased risk for developing breast cancer (DeCensi et al., 2015). Women who have been determined to have a 20%–25% chance of developing breast cancer based on cancer risk assessment tools are considered to be at high risk. A family history of first-degree relatives with breast cancer, known or suspected BRCA1 or BRCA2 variants, familial hereditary cancer syndromes (e.g., Li–Fraumeni), and prior radiation to the chest wall increase women’s lifetime risk of developing breast cancer (American Cancer Society, 2022; Thorat & Balasubramanian, 2020; Visvanathan et al., 2013).
Treatment options for women who are considered to have an elevated risk of developing breast cancer include lifestyle modification, genetic counseling, magnetic resonance imaging surveillance beginning at age 20–30 years, and risk-reducing surgeries (bilateral risk-reducing mastectomy, bilateral risk-reducing salpingo-oophorectomy) (Thorat & Balasubramanian, 2020). In addition, some women elect to use chemoprevention as a risk-reducing strategy.
The U.S. Preventive Services Task Force (2019) recommends that clinicians offer risk-reducing medications such as tamoxifen, raloxifene, or aromatase inhibitors to women who have an increased risk of developing breast cancer and a low risk of experiencing medication side effects. Aromatase inhibitors such as exemestane and anastrozole decrease the amount of available estrogen, and selective estrogen receptor modulators (tamoxifen, raloxifene) block estrogen receptors in breast tissue to prevent tumorigenesis (National Comprehensive Cancer Network, 2022; Thorat & Balasubramanian, 2020). These medications are estimated to reduce the incidence of breast cancer by 43%–65% in high-risk women when taken for five years (Cuzick et al., 2014, 2015; Goss et al., 2011). However, research indicates that the uptake of antiestrogens among eligible women ranges from 5% to 16.3% (Aktas et al., 2016; Crew, 2015; Donnelly et al., 2014; Ropka et al., 2010; Smith et al., 2016) because of concerns about adverse events, side effects, and symptom burden, which may include vasomotor symptoms, weight gain, sexual dysfunction, depression, and increased risk of clot formation and endometrial cancer (Hamer et al., 2017). Because antiestrogen medications are administered daily, they serve as a constant reminder of breast cancer risk, which may contribute to low uptake among high-risk women (Donnelly et al., 2014).
Denosumab, a fully humanized monoclonal antibody that binds to receptor activator of nuclear factor kappa-B ligand, has a potential role in the prevention of breast cancers that are estrogen receptor–, progesterone receptor–, and HER2 protein–negative (i.e., triple-negative breast cancers), for which there are currently no effective chemoprevention methods (Crew, 2015; Evans et al., 2018; Hwang et al., 2019; Toriola et al., 2017, 2018). In addition, denosumab can be given as a depot subcutaneous injection once every six months (Scott & Muir, 2011) and has infrequent side effects (Gnant et al., 2016; Kendler et al., 2011), features that may result in better rates of uptake among at-risk women than have been observed with antiestrogen therapy.
Based on the low rates of at-risk women taking antiestrogen therapy and the investigation of novel breast cancer chemoprevention agents such as denosumab, it is clear that determining the factors that promote the uptake of chemoprevention among eligible women is key to ensuring that they receive the ongoing benefits of chemoprevention. Understanding the health beliefs of women who are at high risk for breast cancer and delineating the barriers to the uptake of chemoprevention are high-priority research goals that will ultimately improve women’s health. Therefore, the purpose of this study was to examine the relationship between women’s health beliefs and their intention to take breast cancer chemoprevention. Specifically, the authors aimed to analyze the relationships between women’s reported levels of breast cancer fatalism, fear, perceived threat, benefits, barriers, and self-efficacy and women’s intention to take breast cancer chemoprevention.
Theoretical Framework
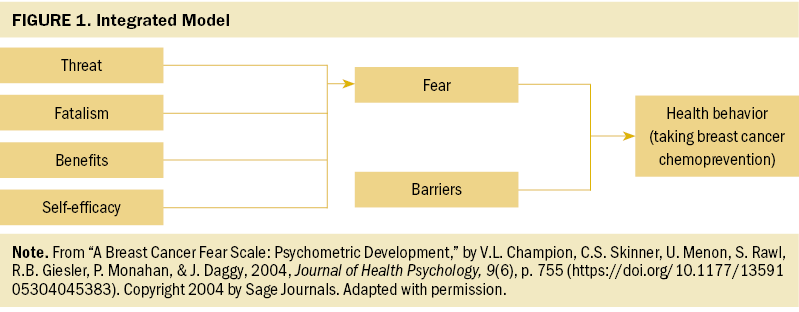
The theoretical framework that guided this research was proposed by Champion et al. (2004). This framework combines concepts of self-efficacy, perceived threat, benefits, and barriers from the Health Belief Model (Strecher & Rosenstock, 1997) with fear from the extended parallel process model (Witte, 1992) and cancer fatalism (Powe, 1995) to predict engagement in mammography screening. The integrated model proposes that fear of breast cancer and barriers to action (e.g., burdens of taking medications) can explain health behaviors (e.g., mammography uptake) (see Table 1 and Figure 1).
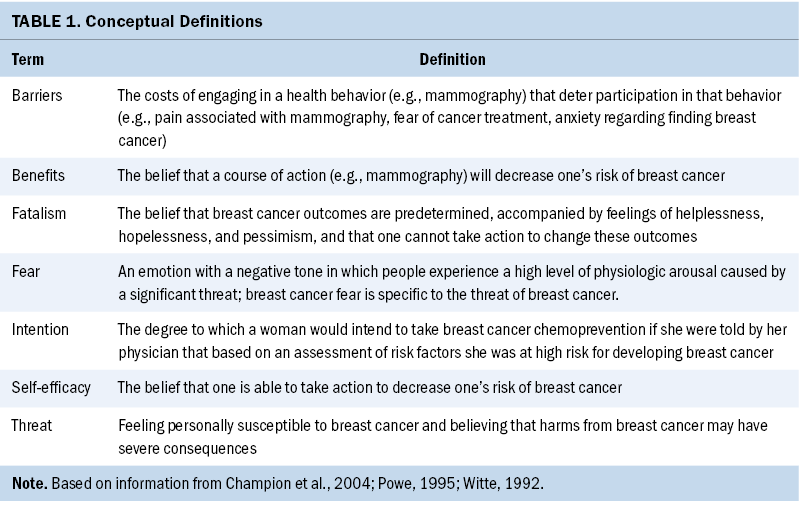
Health Belief Model concepts have been widely applied in the field of breast cancer research to explain the factors that lead women to adopt healthy behaviors surrounding breast cancer screening and prevention. Researchers have found that higher degrees of self-efficacy, greater perceived benefits of screening, and more severe perceived threat of breast cancer, as well as fewer barriers to seeking health care, confer better engagement in mammography screening (Bennett et al., 2010; Champion, 1999; Champion et al., 2005; Laing & Makambi, 2008; VanDyke & Shell, 2017), breast self-examination (Al-Sakkaf & Basaleem, 2016; Champion & Scott, 1997), clinical breast examination (Lee et al., 2015), BRCA gene variant testing (Manchanda et al., 2019), and risk-reducing surgical interventions such as mastectomy and oophorectomy (Ladd et al., 2020). Other researchers have evaluated the role that breast cancer fear (Ackerson & Preston, 2009; Champion et al., 2004) and cancer fatalism (Lopez-McKee et al., 2008; Mayo et al., 2001) play in the uptake of breast cancer screening. However, little research has been conducted to determine whether these health beliefs, which are salient to the uptake of other breast cancer preventive behaviors, could also explain the uptake of breast cancer chemoprevention.
Sample and Setting
Postmenopausal women (N = 400) were recruited from the Joanne Knight Breast Health Center at the Siteman Cancer Center at the Washington University School of Medicine in St. Louis, Missouri, between October 2017 and September 2018 for a study on mammographic breast density (R21 CA216515). Women who were aged 50–64 years and had no prior history of cancer were contacted before their routine screening mammography appointment to determine their interest in participating in this study. Women with a history of breast augmentation, reduction, or implants were ineligible. In addition, women who had taken medication to reduce their risk of breast cancer in the past six months or who were taking hormone replacement therapy were excluded.
Methods and Variables
All study protocols and procedures were approved by the Washington University Institutional Review Board. Women who agreed to participate in the mammographic breast density study completed the informed consent process upon arriving at their routine mammography appointment. Women who wanted to proceed with the study completed a mammogram and questionnaires to collect demographic data and data regarding breast cancer health beliefs. All study procedures took about 45–60 minutes to complete. Once the women had completed the study, they were given a $30 gift card.
Instruments
To operationalize the health beliefs that theoretically influence women’s intention to use breast cancer chemoprevention (breast cancer fatalism, fear, perceived threat, benefits, barriers, and self-efficacy), the authors used established measures and adapted existing measures. To the authors’ knowledge, there are no instruments available to measure health beliefs specifically regarding breast cancer chemoprevention. Although many of these scales have been evaluated previously in the context of mammography (Champion, 1999; Champion et al., 2004, 2005; Lopez-McKee et al., 2008; Mayo et al., 2001), they have never been evaluated in reference to chemoprevention. Thus, the authors have adapted many of the scales and included novel items to make them relevant to this study. In addition, the authors conducted preliminary psychometric evaluations of the adapted instruments to determine reliability and dimensionality (see Table 2).
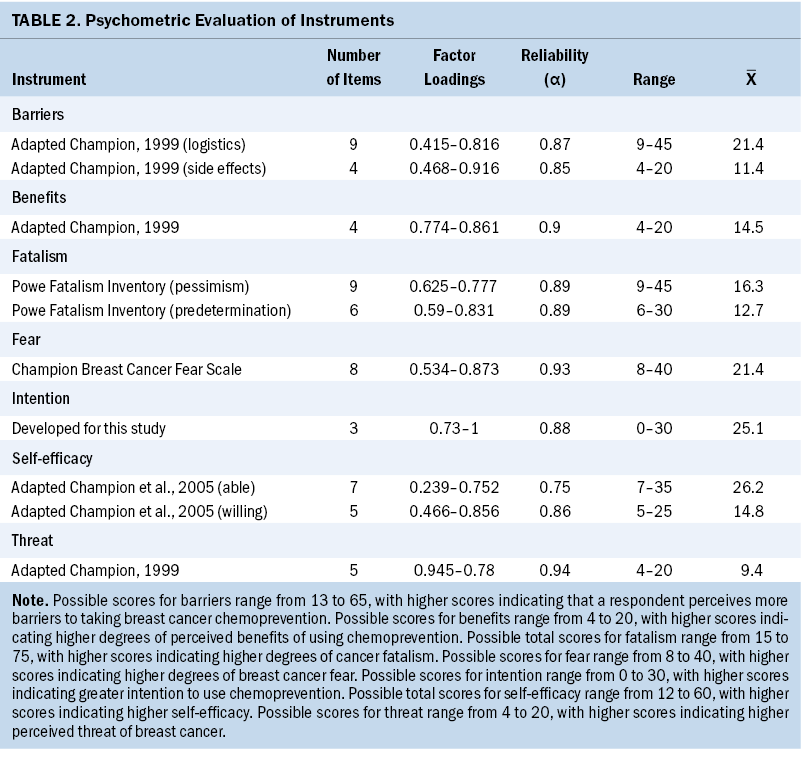
Powe Fatalism Inventory: The Powe Fatalism Inventory is a 15-item instrument used to operationalize cancer fatalism (Powe, 1995). Respondents are instructed to identify the degree to which they agree or disagree with statements such as “I often feel helpless dealing with the problems of life.” Although the original scoring method for this instrument was dichotomous, the authors scored the items on a five-point Likert-type scale, with possible scores for items ranging from 1 (strongly disagree) to 5 (strongly agree), which is a scoring method that has been used and tested by other researchers (Cobran et al., 2014; Lee & Lee, 2018; Shen et al., 2009). In addition, researchers have used the Powe Fatalism Inventory with this scoring method to assess breast cancer fatalism (Lopez-McKee et al., 2008; Mayo et al., 2001). Using the five-point scoring method, total scores can range from 15 to 75, with higher scores indicating higher degrees of cancer fatalism.
Champion Breast Cancer Fear Scale: Women in this sample also completed the Champion Breast Cancer Fear Scale (Champion et al., 2004). This scale has eight items, which include statements such as “The thought of breast cancer scares me.” Items are scored on a five-point Likert-type scale, and possible answers range from 1 (strongly disagree) to 5 (strongly agree). None of the items are reverse scored. Possible scores range from 8 to 40, with higher scores indicating higher degrees of breast cancer fear.
Threat: Perceived threat of breast cancer was measured using an adaption of Champion’s (1999) revised susceptibility, benefits, and barriers scale for mammography screening. Three items are the same as Champion’s original items, but the authors added one novel item to make this scale relevant to chemoprevention, which states, “I have risk factors that make it likely that I will get breast cancer.” Respondents were asked to indicate the degree to which they believe they are susceptible to getting breast cancer on a scale from 1 (strongly disagree) to 5 (strongly agree). Possible scores range from 4 to 20, with higher scores indicating higher perceived threat of breast cancer.
Benefits: The benefits scale was adapted from Champion’s (1999) revised susceptibility, benefits, and barriers scale for mammography screening. The original benefits subscale contained five items, but the current authors’ adaptation included four items. The authors also adapted items to address taking medication to reduce the risk of breast cancer. Benefits were operationalized by asking participants to indicate the degree to which they agree or disagree with statements such as “If I could use a medicine, it would be an easy way to prevent breast cancer.” The authors scored the items on a five-point Likert-type scale from 1 (strongly disagree) to 5 (strongly agree). Possible scores range from 4 to 20, with higher scores indicating higher degrees of perceived benefits of using chemoprevention.
Barriers: The barriers scale was adapted from Champion’s (1999) revised susceptibility, benefits, and barriers scale for mammography screening. Because many of the original items were specific to mammography, the authors changed the wording of 6 items and created 7 novel items, resulting in a total of 13 items. The authors operationalized barriers by asking respondents to indicate the degree to which they agreed or disagreed with statements such as “Medicines are too expensive to take for prevention.” Other items on the scale pertain to logistic barriers such as transportation, making appointments, and being able to get medications. Items are scored on a five-point Likert-type scale from 1 (strongly disagree) to 5 (strongly agree). Possible scores range from 13 to 65, with higher scores indicating that a respondent perceives more barriers to taking breast cancer chemoprevention.
Self-efficacy: The self-efficacy scale was adapted from a self-efficacy scale for mammography described by Champion et al. (2005). The original scale consisted of 10 items. The authors’ adaptation resulted in 12 items, 9 of which are similar in language to the original scale, with appropriate revisions to support the change in target behavior to using chemoprevention. The authors operationalized self-efficacy by asking respondents to indicate the degree to which they agree or disagree with statements such as “You would find a way to pay for a breast cancer prevention medicine.” Possible scores range from 12 to 60, with higher scores indicating higher self-efficacy.
Intention: The authors generated three novel items to operationalize women’s intention to use breast cancer chemoprevention. In each item, respondents were asked to indicate their level of interest in taking a medicine to prevent breast cancer on a scale from 0 (not at all interested) to 10 (extremely interested). Scores could range from 0–30, with higher scores indicating greater intention.
Data Analysis
After ensuring that the data met statistical assumptions, the authors regressed intention to take breast cancer chemoprevention (the outcome variable) onto the health belief scores (breast cancer fatalism, fear, perceived threat, benefits, barriers, and self-efficacy; the predictor variables). The authors used two-sided statistical tests with a level set to 0.05 for determining statistical significance. Slope coefficients (unstandardized B and beta) and confidence intervals (CIs) were examined to determine whether the independent variables in the study’s model significantly predicted the outcome variable. IBM SPSS, version 26.0, was used to conduct all statistical analyses.
Results
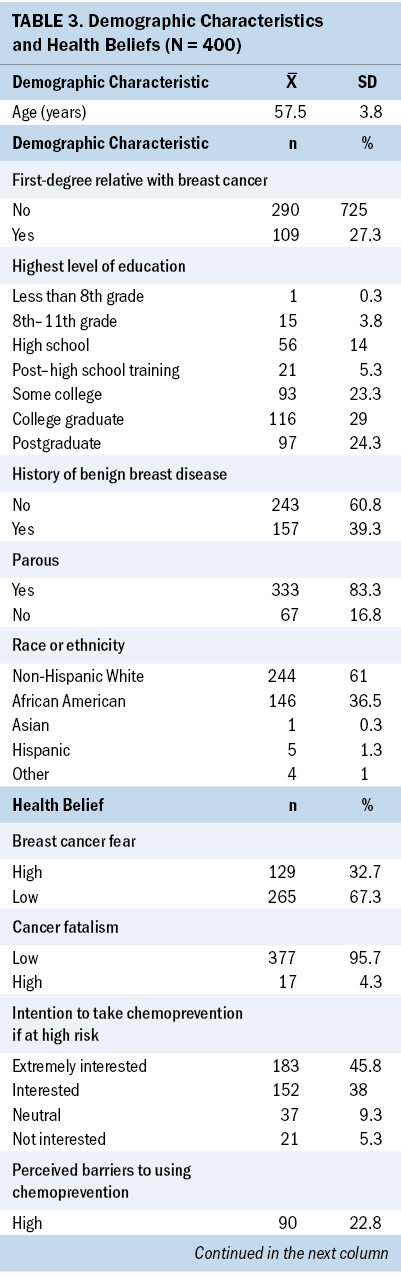
The mean age of study participants (N = 400) was 57.5 years (SD = 3.8). Most participants were non-Hispanic White women (61%) who had graduated from college (53%); 243 women (61%) had no history of benign breast disease. Nearly half (n = 183, 46%) indicated that they would be extremely interested in using chemoprevention, and only a few (n = 21, 5%) indicated that they would not be interested in taking breast cancer chemoprevention (see Table 3).
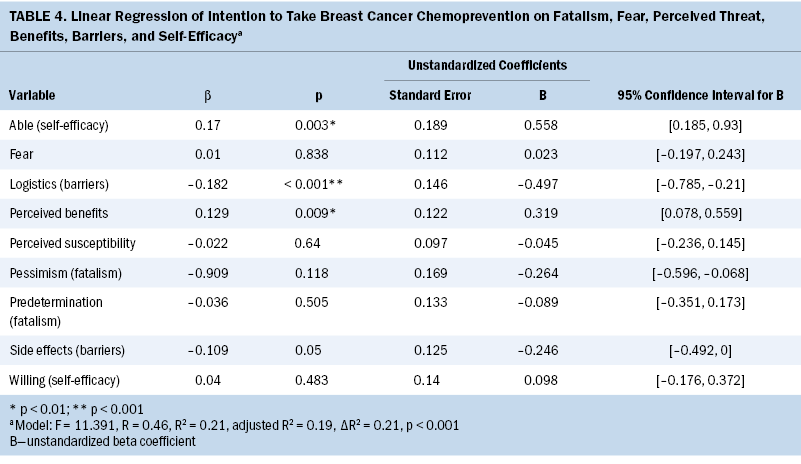
The authors found that women who reported higher perceived benefits of chemoprevention (unstandardized B = 0.32; beta = 0.129; p = 0.009; 95% CI [0.078, 0.559]), higher perceptions of their ability to use chemoprevention (self-efficacy; unstandardized B = 0.56; beta = 0.17; p = 0.003; 95% CI [0.185, 0.93]), and fewer logistic barriers to seeking health care (unstandardized B = –0.5; beta = –0.182; p < 0.001; 95% CI [–0.785, –0.21]) had significantly higher intention to use chemoprevention if they were found to be at high risk for developing breast cancer (F = 11.391, adjusted R2 = 0.193, p < 0.001). The authors found a relationship between barriers resulting from concerns about side effects and intention; however, this relationship was not statistically significant (p = 0.05) (see Table 4). Breast cancer fear, fatalism, and perceived threat were not significantly related to women’s intention to take breast cancer chemoprevention.
Discussion
Logistic barriers, self-efficacy to overcome barriers to seeking health care, and perceived benefits of chemoprevention were strongly related to women’s intention to use chemoprevention. Specifically, logistic barriers (e.g., transportation, routine, cost, insurance, scheduling, navigating the healthcare system, competing demands) were negatively related to intention to use chemoprevention. Self-efficacy (e.g., ability to get transportation, schedule appointments, navigate the healthcare system, and pay for and get medication) was positively related to intention to use chemoprevention. In addition, perceived benefits of using chemoprevention (i.e., the perception that breast cancer risk is effectively reduced by using chemoprevention) were positively related to intention to use chemoprevention. Thus, practical issues that prevent (barriers) or promote (self-efficacy) women’s uptake of chemoprevention and women’s perception that chemoprevention will help reduce their risk of breast cancer (benefits) are salient to its uptake.
The few researchers who have analyzed the barriers to using chemoprevention have found that lack of information regarding chemoprevention; uncertainty regarding taking a medication when one is in generally good health; and concerns regarding the cost of treatment, side effects, adverse events, competing demands, and inconveniences of taking medication impeded women’s intention to use chemoprevention (Bober et al., 2004; Brewster et al., 2012; Cyrus-David & Strom, 2001; Heisey et al., 2006). In addition, researchers have demonstrated that women with lower incomes and lower levels of education are less likely to take breast cancer chemoprevention (Fasching et al., 2007; Melnikow et al., 2005; Tija et al., 2008). Similarly, acceptance of chemoprevention among low-income women was related to the cost of and insurance coverage for the medication (Cyrus-David & Strom, 2001; Salant et al., 2006). Sometimes women confuse chemoprevention with chemotherapy (Cyrus-David & Strom, 2001), indicating that the term “chemoprevention” itself may serve as a barrier to its uptake (Crew, 2015; Cuzick et al., 2011; Heisey et al., 2006).
The authors found evidence that as the perceived benefits of using chemoprevention increased, there was a statistically significant increase in women’s intention to use chemoprevention. Other researchers similarly have found that greater perceived benefits were associated with greater intention to use chemoprevention (Conley et al., 2019; Ralph et al., 2014). In addition, in a sample of high-risk women, Bober et al. (2004) found that those who declined tamoxifen felt more strongly that the medication would not prevent cancer than women who decided to take tamoxifen. This suggests that measures to enhance women’s education regarding chemoprevention effectiveness could help increase its uptake among at-risk women. Evidence-based, accurate information about the efficacy of chemoprevention based on risk stratification is critical for women when deciding on a risk management option (Ralph et al., 2014).
Selecting a breast cancer risk management option is challenging for patients and healthcare providers alike. Because there are no biomarkers available to demonstrate that chemoprevention is reducing a woman’s risk of developing breast cancer, it is difficult to demonstrate that chemoprevention measures are having an effect (Crew, 2015). For example, patients who take medications to lower their cholesterol levels can see that their cholesterol decreases over time. However, there is no comparable laboratory value to demonstrate to at-risk women that chemoprevention is having an effect (DeCensi et al., 2015). In addition, risk prediction models are not always accurate; it is possible that women who would have never developed breast cancer in the first place could use chemoprevention. Although chemoprevention reduces the risk of developing breast cancer, there is no evidence suggesting that there is a mortality benefit for high-risk women using chemoprevention (DeCensi et al., 2015). Putting women at risk for complications from chemoprevention that may be unnecessary further obscures the perception that it is beneficial.
Because it is challenging to ascertain whether risk reduction is occurring when using chemoprevention, many researchers have found that recommendations and communication from healthcare providers are influential factors in women’s decisions to start chemoprevention (Bober et al., 2004; Crew, 2015; Cyrus-David & Strom, 2001; Heisey et al., 2006). Research has found that decision aids are not helpful to women weighing the pros and cons of using chemoprevention and deciding between risk modification strategies (Brewster et al., 2012). In one sample, 40% of women reported that having a provider recommend chemoprevention was influential in their decision to use it (Bober et al., 2004). Many providers report that they wait for women to ask about chemoprevention (Keogh et al., 2009). However, breast cancer pathogenesis, the mechanism of action of chemoprevention, and breast cancer risk stratification are not concepts about which laywomen can be expected to have common knowledge, making a provider’s recommendation for chemoprevention a crucial part of the decision to start.
Interestingly, nearly 84% of women in this study’s sample (who were at average risk for breast cancer) indicated that they would use chemoprevention if a physician told them they were at high risk for developing breast cancer. However, other researchers have reported that 12%–45% of women with known risk factors stated they would be willing to use chemoprevention if indicated (Cyrus-David & Strom, 2001; Meiser et al., 2003; Ralph et al., 2014). This study’s finding is unexpected, given that women at higher risk for breast cancer might be more intentional about using chemoprevention.
The authors posit that mammography screening appointments may be the ideal encounter to discuss chemoprevention. Women in this study’s sample who were seeking mammography tended to have high self-efficacy and fewer barriers to health care and to perceive high benefits of using chemoprevention.
Women may demonstrate more readiness to engage in conversations about chemoprevention at their breast health or obstetric/gynecologic appointments because they may be optimally motivated to prevent breast cancer. In addition, obstetric/gynecologic providers may have more experience prescribing chemoprevention than individuals working in family medicine or internal medicine (Kaplan et al., 2005), so they may be the ideal providers to oversee these patient encounters. Another explanation for women’s high intention to use chemoprevention may be that women in this study’s sample tended to have high levels of education. More than half (n = 213) of participants indicated that they had a college degree or had completed postgraduate education. Other researchers have reported that mammography nonadherence is positively related to lower levels of education (Dailey et al., 2007). There are no research findings to suggest a relationship between the level of education and uptake of chemoprevention. However, the health beliefs that are related to intention to use chemoprevention in this study’s sample (self-efficacy, logistic barriers, and perceived benefit) have been linked to level of education by other researchers (Champion & Menon, 1997). Thus, women with higher education levels might be more motivated to take breast cancer chemoprevention if recommended by their healthcare provider.
Limitations
Limitations of this study include having a homogeneous sample of women who tended to be well educated and who were already engaged in breast health maintenance (mammography). Thus, future researchers might consider sampling women with more heterogeneity regarding their adherence to mammography screening recommendations. Another limitation is that the authors asked women to hypothetically consider whether they would intend to use chemoprevention if they were determined to be at high risk. However, women in the study sample were considered to have an average risk of developing breast cancer. Intentions are antecedent to behavior change, but it cannot be extrapolated that women would elect to use chemoprevention in a real-world scenario. Future researchers might consider measuring the actual uptake of chemoprevention as an outcome, rather than merely intention to use chemoprevention. In addition, this study only considers women’s intention to use chemoprevention for breast cancer. Yet there are many options for risk management, including risk-reducing surgeries and frequent surveillance. Therefore, the authors are unable to offer a comprehensive view of women’s intentions to engage in all breast cancer risk–reducing behaviors, which is a limitation of this study. In addition, women at high risk for breast cancer can be as young as 35 years, so the age range of women in this study (50–64 years) may limit the generalizability of the current findings to younger women who are eligible to take breast cancer chemoprevention. This study’s findings should be considered within the context of these limitations.
Strengths
The strengths of the current study include that it provides preliminary evidence of the theoretical propositions that apply specifically to the health behavior of taking breast cancer chemoprevention. The importance of this research is underscored by the fact that the uptake of chemoprevention has been reported to be quite low in the past (Crew, 2015; Donnelly et al., 2014; Ropka et al., 2010). Limited use of chemoprevention agents, in turn, jeopardizes future research and new developments in the field of chemoprevention, including further study of novel agents such as denosumab and other antiestrogens with more favorable side effect profiles than current U.S. Food and Drug Administration–approved options for preventive therapy. Further research is needed to adequately delineate the health beliefs that influence the uptake of chemoprevention because this knowledge can guide the development of interventions designed to optimize its use. To the authors’ knowledge, this study is the first of its kind to provide preliminary evidence that women at their routine screening mammography appointments are receptive to the possibility of taking breast cancer chemoprevention if they are found to be at high risk for breast cancer.
Implications for Nursing
Barriers to navigating the healthcare system, such as transportation, cost of medication, and scheduling medical appointments, are ubiquitous impediments to seeking health care in general and seem to be a substantial determinant of using chemoprevention. Efforts to reduce these barriers will likely require healthcare systems to strengthen their patient navigation programs. Patient navigators are an effective means to help patients schedule appointments, get medications, and follow up to assess for the occurrence of side effects and continue medication teaching as needed. Patients with cancer who are placed with nurse navigators report higher quality of life and have fewer hospitalizations than those without nurse navigators (Lee et al., 2011). Other ways to reduce patient barriers to health care could include interventions as simple as offering free parking or shuttle services (Solomon et al., 2020). On-site pharmacies reduce the need for patients to make an additional trip to get medications after seeing a provider, thereby facilitating the process of procuring medications and often reducing their cost (Wright et al., 2016). The barriers to seeking health care are extensive and will require healthcare systems to invest in programs to make navigating easier, ultimately reducing the public health burden of breast cancer. In addition, a more comprehensive strategy for educating women about their breast cancer risk and weighing the risks and benefits of treatment may be accomplished through delegating risk stratification and decisions regarding chemoprevention to healthcare providers working in breast health clinics or obstetric/gynecologic clinics.
In addition, the authors’ findings indicate that women may be optimally motivated to learn about and consider using chemoprevention during their routine mammography screening. Screening mammography does not often include physician consultation. However, restructuring routine screening mammography encounters to include a brief screening tool for determining risk and, if indicated, counseling regarding the risks and benefits of using chemoprevention could be beneficial. Suboptimal insurance reimbursement for breast cancer chemoprevention counseling has been identified as an impediment to educating women (Ralph et al., 2014); ensuring better coverage for counseling services is essential to providing women with the opportunity to learn about their options for breast cancer risk reduction.

Conclusion
The uptake of chemoprevention among women at risk for breast cancer is suboptimal, and little research has been done to determine the factors that influence it. The authors found that barriers to seeking health care and self-efficacy to cope with those barriers strongly predicted women’s intention to take breast cancer chemoprevention. Perceived benefits of using chemoprevention were also positively related to intention to use chemoprevention. Thus, interventions aimed at reducing barriers to health care and enabling ample provider time and resources to educate patients about chemoprevention stand to increase its uptake among at-risk women. In addition, women at the time of mammography and women with higher levels of education may be motivated to consider using chemoprevention. Future research endeavors and the development of interventions aimed at decreasing barriers to using chemoprevention, improving women’s self-efficacy to overcome those barriers, increasing their perception that chemoprevention will be beneficial, and determining the optimum timing of these interventions can positively influence women’s intention to take breast cancer chemoprevention.
The authors gratefully acknowledge Helen W. Lach, PhD, RN, CNL, FGSA, FAAN, for editing this manuscript and John Taylor, PhD, for his statistical expertise.
About the Authors
Kristin G. Keller, PhD, MS, RN, is an assistant professor in the Trudy Busch Valentine School of Nursing at Saint Louis University, Adetunji T. Toriola, MD, PhD, MPH, is a professor of surgery in the Division of Public Health Sciences in the Mary Culver Department of Surgery at the Alvin J. Siteman Cancer Center in the School of Medicine at Washington University in St. Louis, and Joanne Kraenzle Schneider, PhD, RN, is a professor in the Trudy Busch Valentine School of Nursing, all in Missouri. This research was funded, in part, by a National Institutes of Health grant (R21CA216515 and R37CA235602). Toriola and Schneider contributed to the conceptualization and design. Toriola completed the data collection. Schneider provided statistical support. Keller provided the analysis. All authors contributed to the manuscript preparation. Keller can be reached at kristin.keller@slu.edu, with copy to ONFEditor@ons.org. (Submitted November 2022. Accepted April 21, 2023.)
References
Ackerson, K., & Preston, S.D. (2009). A decision theory perspective on why women do or do not decide to have cancer screening: Systematic review. Journal of Advanced Nursing, 65(6), 1130–1140. https://doi.org/10.1111/j.1365-2648.2009.04981.x
Aktas, B., Sorkin, M., Pusztai, L., & Hofstatter, E.W. (2016). Uptake of exemestane chemoprevention in postmenopausal women at increased risk for breast cancer. European Journal of Cancer Prevention, 25(1), 3–8. https://doi.org/10.1097/cej.0000000000000124
Al-Sakkaf, K.A., & Basaleem, H.O. (2016). Breast cancer knowledge, perception and breast self-examination practices among Yemeni women: An application of the health belief model. Asian Pacific Journal of Cancer Prevention, 17(3), 1463–1467. https://doi.org/10.7314/apjcp.2016.17.3.1463
American Cancer Society. (2022). American Cancer Society recommendations for the early detection of breast cancer. https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-d…
American Cancer Society. (2023). Key statistics for breast cancer. https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-…
Bennett, P., Parsons, E., Brain, K., & Hood, K. (2010). Long-term cohort study of women at intermediate risk of familial breast cancer: Experiences of living at risk. Psycho-Oncology, 19(4), 390–398. https://doi.org/10.1002/pon.1588
Bober, S.L., Hoke, L.A., Duda, R.B., Regan, M.M., & Tung, N.M. (2004). Decision-making about tamoxifen in women at high risk for breast cancer: Clinical and psychological factors. Journal of Clinical Oncology, 22(24), 4951–4957. https://doi.org/10.1200/jco.2004.05.192
Brewster, A.M., Davidson, N.E., & McCaskill-Stevens, W. (2012). Chemoprevention for breast cancer: Overcoming barriers to treatment. American Society of Clinical Oncology Educational Book, 32, 85–90. https://doi.org/10.14694/edbook_am.2012.32.152
Champion, V.L. (1999). Revised susceptibility, benefits, and barriers scale for mammography screening. Research in Nursing and Health, 22(4), 341–348. https://doi.org/10.1002/(sici)1098-240x(199908)22:4%3C341::aid-nur8%3E3…
Champion, V.L., & Menon, U. (1997). Predicting mammography and breast self-examination in African American women. Cancer Nursing, 20(5), 315–322. https://doi.org/10.1097/00002820-199710000-00002
Champion, V.L., & Scott, C.R. (1997). Reliability and validity of breast cancer screening belief scales in African American women. Nursing Research, 46(6), 331–337. https://doi.org/10.1097/00006199-199711000-00006
Champion, V.L., Skinner, C.S., & Menon, U. (2005). Development of a self-efficacy scale for mammography. Research in Nursing and Health, 28(4), 329–336. https://doi.org/10.1002/nur.20088
Champion, V.L., Skinner, C.S., Menon, U., Rawl, S., Giesler, R.B., Monahan, P., & Daggy, J. (2004). A breast cancer fear scale: Psychometric development. Journal of Health Psychology, 9(6), 753–762. https://doi.org/10.1177/1359105304045383
Cobran, E.K., Wutoh, A.K., Lee, E., Odedina, F.T., Ragin, C., Aiken, W., & Godley, P.A. (2014). Perceptions of prostate cancer fatalism and screening behavior between United States-born and Caribbean-born Black males. Journal of Immigrant and Minority Health, 16(3), 394–400. https://doi.org/10.1007/s10903-013-9825-5
Conley, C.C., Agnese, D.M., Vadaparampil, S.T., & Andersen, B.L. (2019). Factors associated with intentions for breast cancer risk management: Does risk group matter? Psycho-Oncology, 28(5), 1119–1126. https://doi.org/10.1002/pon.5066
Crew, K.D. (2015). Addressing barriers to uptake of breast cancer chemoprevention for patients and providers. American Society of Clinical Oncology Educational Book, e50–e58. https://doi.org/10.14694/edbook_am.2015.35.e50
Cuzick, J., DeCensi, A., Arun, B., Brown, P.H., Castiglione, M., Dunn, B., . . . Zwierzina, H. (2011). Preventive therapy for breast cancer: A consensus statement. Lancet Oncology, 12(5), 496–503. https://doi.org/10.1016/S1470-2045(11)70030-4
Cuzick, J., Sestak, I., Cawthorn, S., Hamed, H., Holli, K., Howell, A., & Forbes, J.F. (2015). Tamoxifen for the prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncology, 16(1), 67–75. https://doi.org/10.1016/s1470-2045(14)71171-4
Cuzick, J., Sestak, I., Forbes, J.F., Dowsett, M., Knox, J., Cawthorn, S., . . . Howell, A. (2014). Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): An international, double-blind, randomized placebo-controlled trial. Lancet, 383(9922), 1041–1048. https://doi.org/10.1016/s0140-6736(13)62292-8
Cyrus-David, M.S., & Strom, S.S. (2001). Chemoprevention of breast cancer with selective estrogen receptor modulators: Views from broadly diverse focus groups of women with elevated risk for breast cancer. Psycho-Oncology, 10(6), 521–533. https://doi.org/10.1002/pon.547
Dailey, A.B., Kasl, S.V., Holford, T.R., Calvocoressi, L., & Jones, B.A. (2007). Neighborhood-level socioeconomic predictors of nonadherence to mammography screening guidelines. Cancer Epidemiology, Biomarkers and Prevention, 16(11), 2293–2303. https://doi.org/10.1158/1055-9965.epi-06-1076
DeCensi, A., Thorat, M.A., Bonanni, B., Smith, S.G., & Cuzick, J. (2015). Barriers to preventive therapy for breast and other major cancers and strategies to improve uptake. Ecancermedicalscience, 9, 595. https://doi.org/10.3332/ecancer.2015.595
Donnelly, L.S., Evans, D.G., Wiseman, J., Fox, J., Greenhalgh, R., Affen, J., . . . Howell, A. (2014). Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic. British Journal of Cancer, 110(7), 1681–1687. https://doi.org/10.1038/bjc.2014.109
Evans, D.G., Howell, S.J., & Howell, A. (2018). Personalized prevention in high risk individuals: Managing hormones and beyond. Breast, 39, 139–147.
Fasching, P.A., von Minckwitz, G., Fischer, T., Kaufmann, M., Schultz-Zehden, B., Beck, H., . . . Paepke, S. (2007). The impact of breast cancer awareness and socioeconomic status on willingness to receive breast cancer prevention drugs. Breast Cancer Research and Treatment, 101(1), 95–104. https://doi.org/10.1007/s10549-006-9272-2
Gnant, M., Pfeiler, G., Dubsky, P.C., Hubalek, M., Greil, R., Jakesz, R., . . . Singer, C.F. (2016). The impact of adjuvant denosumab on disease-free survival: Results from 3,425 postmenopausal patients of the ABCSG-18 trial. Cancer Research, 76(Suppl. 4), S2–02. https://doi.org/10.1158/1538-7445.SABCS15-S2-02
Goss, P.E., Ingle, J.N., Alés-Martínez, J.E., Cheung, A.M., Chlebowski, R.T., Wactawski-Wende, J., . . . Richardson, H. (2011). Exemestane for breast-cancer prevention in postmenopausal women. New England Journal of Medicine, 364(25), 2381–2391. https://doi.org/10.1056/nejmoa1103507
Hamer, J., McDonald, R., Zhang, L., Verma, S., Leahey, A., Ecclestone, C., . . . Chow, E. (2017). Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Supportive Care in Cancer, 25(2), 409–419. https://doi.org/10.1007/s00520-016-3417-6
Heisey, R., Pimlott, N., Clemons, M., Cummings, S. & Drummond, N. (2006). Women’s views on chemoprevention of breast cancer: Qualitative study. Canadian Family Physician, 52(5), 624–625.
Howlader, N., Noone, A.M., Krapcho, M., Miller, D., Brest, A., Yu, M., . . . Cronin, K.A. (Eds.). (2020). SEER cancer statistics review (CSR) 1975–2017. National Cancer Institute. U.S. Department of Health and Human Services. https://seer.cancer.gov/csr/1975_2017
Hwang, S.-Y., Park, S., & Kwon, Y. (2019). Recent therapeutic trends and promising targets in triple negative breast cancer. Pharmacology and Therapeutics, 199, 30–57. https://doi.org/10.1016/j.pharmthera.2019.02.006
Kaplan, C.P., Haas, J.S., Pérez-Stable, E.J., Des Jarlais, G., & Gregorich, S.E. (2005). Factors affecting breast cancer risk reduction practices among California physicians. Preventive Medicine, 41(1), 7–15. https://doi.org/10.1016/j.ypmed.2004.09.041
Kendler, D.L., McClung, M.R., Freemantle, N., Lillestol, M., Moffett, A.H., Borenstein, J., . . . Siddhanti, S. (2011). Adherence, preference, and satisfaction of postmenopausal women taking denosumab or alendronate. Osteoporosis International, 22(6), 1725–1735. https://doi.org/10.1007/s00198-010-1378-z
Keogh, L.A., Hopper, J.L., Rosenthal, D., & Phillips, K.-A. (2009). Australian clinicians and chemoprevention for women at high familial risk for breast cancer. Hereditary Cancer in Clinical Practice, 7(1), 9. https://doi.org/10.1186/1897-4287-7-9
Ladd, M.K., Peshkin, B.N., Senter, L., Baldinger, S., Isaacs, C., Segal, H., . . . Schwartz, M.D. (2020). Predictors of risk reducing surgery intentions following genetic counseling for hereditary breast and ovarian cancer. Translational Behavioral Medicine, 10(2), 337–346. https://doi.org/10.1093/tbm/iby101
Laing, S.S., & Makambi, K. (2008). Predicting regular breast cancer screening in African American women with a familial history of breast cancer. Journal of the National Medical Association, 100(11), 1309–1317. https://doi.org/10.1016/S0027-9684(15)31510-8
Lee, H.Y., Stange, M.J., & Ahluwalia, J.S. (2015). Breast cancer screening behaviors among Korean American immigrant women: Findings from the health belief model. Journal of Transcultural Nursing, 26(5), 450–457. https://doi.org/10.1177/1043659614526457
Lee, S.Y., & Lee. E.E. (2018). Access to health care, beliefs, and behaviors about colorectal cancer screening among Korean Americans. Asian Pacific Journal of Cancer Prevention, 19(7), 2021–2027. https://doi.org/10.22034/apjcp.2018.19.7.2021
Lee, T., Ko, I., Lee, I., Kim, E., Shin, M., Roh, S., . . . Chang, H. (2011). Effects of nurse navigators on health outcomes of cancer patients. Cancer Nursing, 34(5), 376–384. https://doi.org/10.1097/ncc.0b013e3182025007
Lopez-McKee, G., McNeill, J.A., Bader J., & Morales, P. (2008). Comparison of factors affecting repeat mammography screening of low-income Mexican American women. Oncology Nursing Forum, 35(6), 941–947. https://doi.org/10.1188/08.ONF.941-947
Manchanda, R., Burnell, M., Gaba, F., Sanderson, S., Loggenberg, K., Gessler, S., . . . Jacobs, I. (2019). Attitude towards and factors affecting uptake of population-based BRCA testing in the Ashkenazi Jewish population: A cohort study. British Journal of Obstetrics and Gynaecology, 126(6), 784–794. https://doi.org/10.1111/1471-0528.15654
Mayo, R.M., Ureda, J.R., & Parker, V.G. (2001). Importance of fatalism in understanding mammography screening in rural elderly women. Journal of Women and Aging, 13(1), 57–72. https://doi.org/10.1300/j074v13n01_05
Meiser, B., Butow, P., Price, M., Bennett, B., Berry, G., & Tucker, K. (2003). Attitudes to prophylactic surgery and chemoprevention in Australian women at increased risk for breast cancer. Journal of Women’s Health, 12(8), 769–778.
Melnikow, J., Paterniti, D., Azari, R., Kuenneth, C., Birch, S., Kuppermann, M., . . . Henderson, S. (2005). Preferences of women evaluating risk of tamoxifen (POWER) study of preferences for tamoxifen for breast cancer risk reduction. Cancer, 103(10), 1996–2005. https://doi.org/10.1002/cncr.20981
National Comprehensive Cancer Network. (2022). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast cancer risk reduction [v.1.2023]. www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
Powe, B.D. (1995). Cancer fatalism among elderly Caucasians and African Americans. Oncology Nursing Forum, 22(9), 1355–1359.
Ralph, A.F., Ager, B., Bell, M.L., Collins, I.M., Andrews, L., Tucker, K., . . . Butow, P. (2014). Women’s preferences for selective estrogen reuptake modulators: An investigation using protection motivation theory. Patient Education and Counseling, 96(1), 106–112. https://doi.org/10.1016/j.pec.2014.04.011
Ropka, M.E., Keim, J., & Philbrick, J.T. (2010). Patient decisions about breast cancer chemoprevention: A systematic review and meta-analysis. Journal of Clinical Oncology, 28(18), 3090–3095.
Salant, T., Ganschow, P.S., Olopade, O.I., & Lauderdale, D.S. (2006). “Why take it if you don’t have anything?” Breast cancer risk perceptions and prevention choices at a public hospital. Journal of General Internal Medicine, 21(7), 779–785. https://doi.org/10.1111/j.1525-1497.2006.00461.x
Scott, L.J., & Muir, V.J. (2011). Denosumab: In the prevention of skeletal-related events in patients with bone metastases from solid tumours. Drugs, 71(8), 1059–1069.
Shen, L., Condit, C.M., & Wright, L. (2009). The psychometric property and validation of a fatalism scale. Psychology and Health, 24(5), 597–613. https://doi.org/10.1080/08870440801902535
Smith, S.G., Sestak, I., Forster, A., Partridge, A., Side, L., Wolf, M.S., . . . Cuzick, J. (2016). Factors affecting uptake and adherence to breast cancer chemoprevention: A systematic review and meta-analysis. Annals of Oncology, 27(4), 575–590. http://doi.org/10.1093/annonc/mdv590
Solomon, E.M., Wing, H., Steiner, J.F., & Gottlieb, L.M. (2020). Impact of transportation interventions on health care outcomes: A systematic review. Medical Care, 58(4), 384–391. https://doi.org/10.1097/MLR.0000000000001292
Strecher, V.J., & Rosenstock, I.M. (1997). The health belief model. In K. Glanz, F.M. Lewis, & B. Rimer (Eds.), Health behavior and health education: Theory, research, and practice (2nd ed., pp. 41–59). Jossey-Bass Publishers.
Thorat, M.A., & Balasubramanian, R. (2020). Breast cancer prevention in high-risk women. Best Practice and Research Clinical Obstetrics and Gynaecology, 65, 18–31.
Tija, J., Micco, E., & Armstrong, K. (2008). Interest in breast cancer chemoprevention among older women. Breast Cancer Research and Treatment, 108(3), 435–453. https://doi.org/10.1007/s10549-007-9614-8
Toriola, A.T., Appleton, C.M., Zong, X., Luo, J., Weilbaecher, K., Tamimi, R.M., & Colditz, G.A. (2018). Circulating receptor activator of nuclear factor-kB (RANK), RANK ligand (RANKL), and mammographic density in premenopausal women. Cancer Prevention Research, 11(12), 789–796. https://doi.org/10.1158/1940-6207.capr-18-0199
Toriola, A.T., Dang, H.X., Hagemann, I.S., Appleton, C.M., Colditz, G.A., Luo, J., & Maher, C.A. (2017). Increased breast tissue receptor activator of nuclear factor-kB ligand (RANKL) gene expression is associated with higher mammographic density in premenopausal women. Oncotarget, 8(43), 73787–73792. https://doi.org/10.18632/oncotarget.17909
U.S. Preventive Services Task Force. (2019). Breast cancer: Medication use to reduce risk. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/bre…
VanDyke, S.D., & Shell, M.D. (2017). Health beliefs and breast cancer screening in rural Appalachia: An evaluation of the health belief model. Journal of Rural Health, 33(4), 350–360. https://doi.org/10.1111/jrh.12204
Visvanathan, K., Hurley, P., Bantug E., Brown, P., Col, N.F., Cuzick, J., . . . Lippman, S.M. (2013). Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology, 31(23), 2942–2962. https://doi.org/10.1200/jco.2013.49.3122
Witte, K. (1992). Putting the fear back into fear appeals: The extended parallel process model. Communication Monographs, 59(4), 329–349. https://doi.org/10.1080/03637759209376276
Wright, W.A., Gorman, J.M., Odorzynski, M., Peterson, M.J., Clayton, C. (2016). Integrated pharmacies at community mental health centers: Medication adherence and outcomes. Journal of Managed Care and Specialty Pharmacy, 11, 1330–1336.

