Scoping Review of Nonsurgical, Nonpharmacologic Interventions After Risk Reduction: Improving Quality of Life for Patients With Inherited Cancer Risk
Purpose: Although risk-reduction interventions for inherited cancer can significantly reduce cancer risk, they may also lead to distressing symptoms. It is not well understood how clinicians support patients in managing such concerns. This scoping review describes nonsurgical, nonpharmacologic interventions for adults with inherited cancer risk who have completed risk reduction.
Literature Search: Five publications were identified following a database review for English-language articles published from 2015 to 2020.
Data Evaluation: Sample, content, methods, and outcomes of included interventions are summarized.
Results: The study identified five interventions: (a) a mindfulness-based stress reduction (MBSR) intervention for BRCA1/2 carriers after prophylactic oophorectomy, (b) an MBSR-like program for BRCA1/2 carriers, (c) an MBSR workshop for BRCA1/2 carriers after prophylactic oophorectomy, (d) a three-month diet intervention in adult APC carriers after prophylactic colectomy, and (e) a 12-month lifestyle intervention for BRCA1/2 carriers.
Implications for Nursing: Future research should target a broader variety of heritable cancers to identify effective strategies for addressing challenges with risk-reduction interventions for inherited cancer.
Jump to a section
Decades of research have resulted in the generation of evidence-based guidelines to support risk reduction for individuals with inherited risk for cancer (Daly et al., 2021; Gupta et al., 2019). Well-known clinical examples of surgical risk-reduction interventions include the following: (a) risk-reducing salpingo-oophorectomy for hereditary breast and ovarian cancer (HBOC) syndrome (Daly et al., 2021); (b) prophylactic mastectomy for HBOC syndrome, Li-Fraumani syndrome, and Cowden syndrome (Daly et al., 2021); (c) risk-reducing hysterectomy for Lynch syndrome and Cowden syndrome (Daly et al., 2021; Gupta et al., 2019); (d) prophylactic gastrectomy for familial adenomatous polyposis (FAP) and carriers of a CDH1 pathogenic variant (Stjepanovic et al., 2019); and (e) prophylactic colectomy or proctocolectomy for FAP (Gupta et al., 2019). More recently, studies have identified nonsurgical, pharmacologic interventions, such as aspirin (although optimal dosing is still under investigation) to reduce colon cancer risk in Lynch syndrome (Burn et al., 2020), as well as chemoprevention or oral contraception for HBOC syndrome (Gordhandas et al., 2019).
Risk-reduction interventions decrease cancer risk, leading to improved clinical and mental health outcomes in individuals who are at high risk (Altman et al., 2018). However, the positive effects of risk-reduction procedures and medications may be coupled with negative consequences (see Table 1). 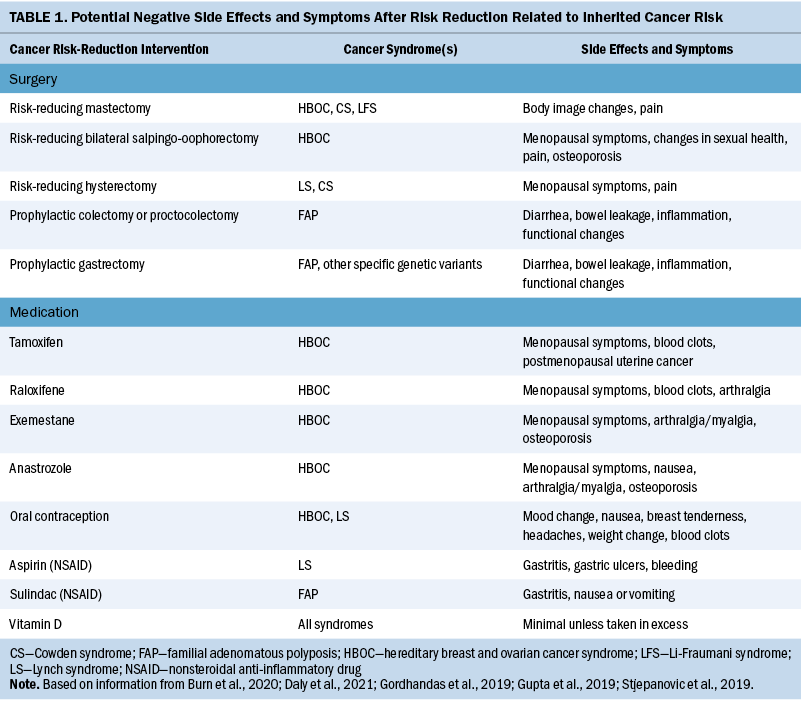
Individuals making risk-reduction decisions about inherited cancer risk face anticipated positive and negative consequences. The experiences of people living with inherited cancer risk differ from those undergoing cancer therapy. These individuals have unique personal and family experiences because of their inherited risk, as well as potential risks for other cancers associated with common interventions, such as the increased risk of breast or uterine cancer resulting from estrogen replacement after removal of ovaries (Vogel et al., 2020). Oncology nurses are at the forefront of the cancer genetics and prevention team. Often, their role includes helping patients and families navigate care. They are poised to support patients by addressing the increased symptom burden that often accompanies the mitigation of heritable cancer risk (Vogel et al., 2020).
Frequently, the responses to these unintended negative consequences are medically focused. For example, clinicians often address menopausal symptoms using hormone replacement therapy (HRT) (Gordhandas et al., 2019), reducing or changing chemoprevention dosages or adding in additional screening recommendations (i.e., uterine cancer screening while in treatment with raloxifene). Addressing such consequences medically can offer some benefit in the reduction of symptoms. However, additional medical or pharmacologic interventions may not be the most welcome response for high-risk patients already inundated with several medical interventions who live in an environment of uncertainty about their future cancer risk (Underhill & Dickerson, 2011). For example, patient willingness to use HRT to treat side effects of risk-reduction surgery is low despite being considered a largely safe and effective treatment to improve menopausal symptoms (Gordhandas et al., 2019).
However, individuals at high risk for cancer do not seek only medical expertise; they also seek self-care strategies to feel as though they can “do something” and take control of cancer risk and their cancer risk experience (Underhill et al., 2012, 2015; Underhill & Crotser, 2014; Underhill & Dickerson, 2011). In addition, there are limits to medical and pharmacologic interventions, and controversy exists in the medical and lay literature about the efficacy and appropriate use of medical interventions, such as HRT for high-risk populations (Birrer et al., 2018). The purpose of this scoping review was to describe existing literature within this domain and identify potential gaps to address through future research and practice initiatives. The research question was as follows: What nonsurgical and nonpharmacologic interventions focused on symptoms and quality of life (QOL) exist for adults with inherited risk who have completed cancer risk reduction?
Methods
A scoping review of the literature was guided by Arksey and O’Malley (2005) and Khalil et al. (2016). Studies were identified in collaboration with a reference librarian. PubMed®, Web of Science, CINAHL®, ClinicalTrials.gov, and Embase® were searched using the following terms: Cowden; Peutz-Jegher; Li-Fraumani; hereditary breast and ovarian cancer; BRCA; familial adenomatous polyposis; hereditary diffuse gastric cancer; Lynch syndrome; inherited cancer risk; and quality of life. Results were limited to citations published in English between 2015 and 2020 to provide current examples of interventions being tested. Interventions designed for children were excluded. The first author led study selection and reviewed and clarified selections with the project team as needed. All titles were screened for inclusion, followed by review of abstracts of all included titles. Full text and reference lists for the selected list of publications were then reviewed to identify any additional references. The first author reviewed and abstracted information about each study that was included in the final sample.
One citation was a ClinicalTrials.gov entry for an inquiry-based stress reduction intervention in BRCA1/BRCA2 pathogenic variant carriers that had no publications listed. However, a manual author search identified one published abstract (Lev-Ari et al., 2014) and two published full-text articles related to the ClinicalTrials.gov entry (Landau et al., 2015, 2016). The published abstract was outside of the time frame of the scoping review (published in 2014), and the results were duplicated in the full-text studies. Therefore, these two studies were included in place of the ClinicalTrials.gov entry. In addition, one Cochrane review (Jeffers et al., 2019) was identified; however, the interventions summarized there were encompassed within the primary studies in this scoping review, so it was excluded.
Data were summarized and reported through detailed tables and narrative summation. Key data points targeted by this review were methods, sample, target cancer syndrome, intervention description, delivery approach, enrollment and completion rates, and summary of outcomes.
Results
Figure 1 describes the review outcomes. A total of 848 citations were screened. Of those, 69 citations were eligible based on the title, and abstracts were screened. The final sample included six studies (Bober et al., 2015; Kiechle et al., 2016; Landau et al., 2015, 2016; Pasanisi et al., 2019; van Driel et al., 2019) describing five unique interventions. 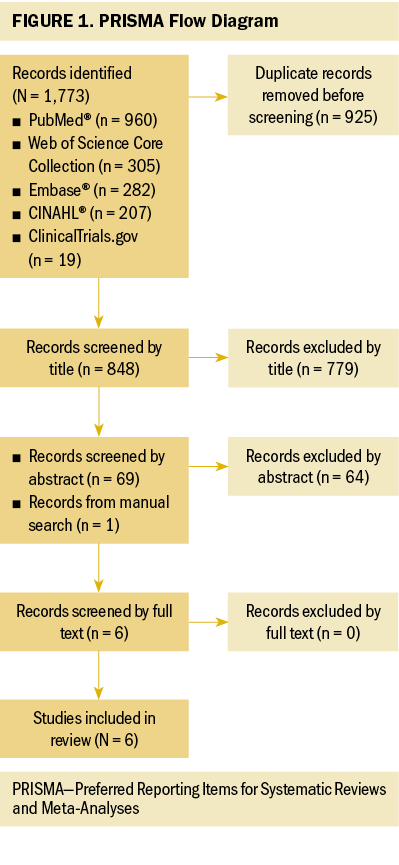
A detailed overview of each study, study participants, and intervention components is presented in Table 2. HBOC was the predominant cancer syndrome in the included studies. Four of the five interventions involved studies with only female patients with HBOC (Bober et al., 2015; Kiechle et al., 2016; Landau et al., 2015, 2016; van Driel et al., 2019). One study included male and female individuals with FAP (Pasanisi et al., 2019). Race and ethnicity were not commonly reported; however, in the one study that did report participants were a majority White (Bober et al., 2015). The sample population of two studies contained people with cancer in addition to people with hereditary risk for cancer (Bober et al., 2015; van Driel et al., 2019). 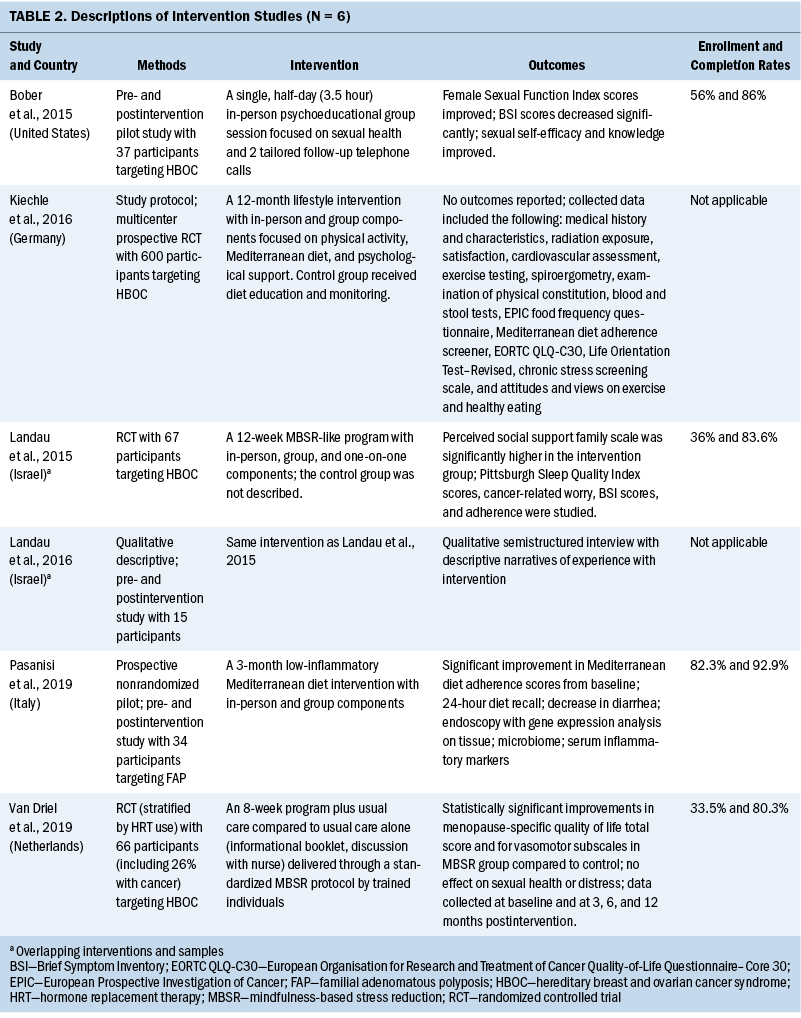
Three of the five interventions were in-person group mindfulness-based stress reduction (MBSR) or MBSR-like programs focused on sexual health, menopausal symptoms, and QOL, with duration ranging from a single session to 12 weeks (Bober et al., 2015; Landau et al., 2015, 2016; van Driel et al., 2019). One intervention was a 12-week in-person group diet intervention focused on improving diarrheal symptoms and QOL (Pasanisi et al., 2019). All interventions included some combination of psychoeducation and home practice activities. Two interventions used one-on-one engagement, either in person or by telephone (Bober et al., 2015; Landau et al., 2015, 2016). Group sessions across all interventions ranged from 2.5 to 3.5 hours in length.
Enrollment rates in non–protocol-based studies were reported and ranged from 33.5% to 82.3% (Pasanisi et al., 2019). However, the latter value (82.3%) is interpreted with caution because in that case, participants were hand selected by the study team. When that study is excluded, the upper range of enrollment was 56%. Attrition ranged from 14% to 27.3% across all interventions.
In the two studies that reported on patient satisfaction, enrolled participants described being satisfied with the program being studied (Bober et al., 2015; Landau et al., 2015). The studies that involved mindfulness-based interventions had positive outcomes for sexual health and function (Bober et al., 2015), menopausal symptoms (van Driel et al., 2019), and perceived family function (Landau et al., 2015). Although the results of the dietary intervention have not yet been fully reported, reports of diarrhea episodes decreased (Pasanisi et al., 2019).
Discussion
This scoping review describes nonpharmacologic and nonsurgical symptom and QOL interventions for individuals with inherited cancer risk who have completed risk-reduction treatment. Studies of five interventions were identified by this scoping review: two were MBSR or MBSR-like interventions focused on sexual health; one was an inquiry-based, stress-reduction meditation focused on overall QOL and psychosocial health; one was a Mediterranean diet intervention focused on overall health and QOL; and one was a study protocol for a lifestyle intervention to reduce stress and improve diet, activity, and QOL. Overall, these are promising interventions to consider in the future; however, more work is needed to fully flesh out their clinical feasibility, as well as the impact of implementing such interventions. In addition, it is important to study these interventions in a more diverse and representative sample of individuals across multiple risk syndromes.
Studies identified by this review were largely overrepresented with White, female individuals with HBOC. The initial literature search included multiple adult cancer syndromes with risk reduction guidelines and recommendations for men and women of all racial and ethnic backgrounds. However, the scoping review did not identify studies for these adult cancer syndromes. Because multigene, multisyndrome genetic testing is now the standard of care, such limited scope interventions do not meet overall patient needs in the current scientific and clinical landscape.
Three of five interventions incorporated some cognitive behavioral therapy–related component such as MBSR or inquiry-based stress reduction. MBSR is an effective standardized approach (typically 2.5 hours over eight weeks) with demonstrated efficacy in the context of cancer (Niazi & Niazi, 2011). However, it may not be feasible, sustainable, or practical to implement such a structured approach on a larger scale, which may be a reason for the relatively small sample sizes of the identified interventions. Considering more flexible types of cognitive behavioral therapy grounded in mindfulness, such as Acceptance and Commitment Therapy (ACT), may benefit future research. ACT is a flexible approach to mindfulness, and when used as a framework, it has demonstrated promise in improving outcomes in the context of psychological distress, grief, and fear (Gonzalez-Fernandez & Fernandez-Rodriguez, 2019). Applying the ACT framework to a high-risk population might be beneficial.
Overall, with the relatively low enrollment rates in the reviewed studies (56% or less), institutions, clinical settings, or agencies must consider whether to adopt nonsurgical, nonpharmacologic interventions as policy if uptake reflects a lack of representative engagement in a wide range of patient populations. This is critical to address if interventions are to be sustained in clinical practice.
One reason for lower-than-expected enrollment may be the time intensity required for in-person prospective group sessions or engagement (Nam & Toneatto, 2016). In a rural or limited resource setting, this barrier may be even more prominent. Concerns related to the sensitive nature of some topic areas, such as sexual health, may also be a barrier to discussing topics in a live group session (Dai et al., 2020).
Considering technology-based live or asynchronous interventions might be one solution. Using technology, people can participate in a more accessible way in groups with a shared experience. Technology also allows for more privacy when sharing sensitive information. Engaging people in the comfort of their private settings might provide enhanced comfort and ease of use, resulting in increased adherence and enrollment success. Previous research has demonstrated that delivery of MBSR or psychoeducational interventions through technology can be effective (Russell et al., 2018; Victorson et al., 2020). Workflows would need to be tailored to each setting to be able to sustain and engage participants in this type of research or clinical offering in a meaningful way (Moulton-Perkins et al., 2020).
Limitations
Studies were limited to English-language studies from the past five years. Therefore, some important publications might have been missed.
The scope of this study was limited to QOL and symptom implications for patients who had undergone risk reduction only. However, nonpharmacologic interventions might be warranted within high-risk populations (e.g., people living with grief and loss, approaching risk, coping with surveillance). Therefore, more research is needed.
In addition, the sample populations in the included studies often contained people with cancer and those with inherited risk for cancer. It is difficult to understand differences in outcomes and experiences between those two distinct groups participating in the same study.
Implications for Nursing
Nurses at all levels who work with and care for individuals and families with inherited cancer risk are at the forefront of helping them to make complicated and informed health decisions. Nurses subsequently live through and manage the adverse effects of those decisions. Cancer prevention care does not stop once prevention through risk reduction is completed. Nurses, particularly those in hereditary cancer care, often work with individuals and families for entire lifetimes as generations of family members engage in cancer surveillance and prevention, and they understand the impact of cancer on these families. Therefore, it is critical for nurses to recognize, validate, and address the patient experience for those who engage in risk reduction while living with inherited cancer risk. Appropriate informed shared decision-making is an important component of facilitating the decision-making process for risk reduction and associated interventions. Risk-reducing behaviors have good and bad outcomes for patients’ lives within and beyond cancer risk, and that is the context in which nursing can provide critical insight into patient care. Research efforts should target the development of scalable interventions that can be practically delivered by nurses at the forefront of care. Research efforts should also focus on self-administered interventions that can be facilitated by nurses to individuals and families to promote self-care and reduce symptoms or QOL burden after risk reduction. 
Conclusion
MBSR could improve menopause-related QOL after prophylactic oophorectomy. Future work may suggest diet as an intervention after prophylactic colectomy. More research is needed to understand the impact of MBSR and inquiry-based stress reduction on sleep and cancer worry; however, it may be useful for improving perceived family support. Published QOL-focused interventions for patients completing risk reduction for all sex and cancer syndromes are limited and should be the target of future research. 
About the Author(s)
Meghan Underhill-Blazey, PhD, APRN, FAAN, is an assistant professor in the School of Nursing and a nurse practitioner in the Hereditary Cancer Program at the Wilmot Cancer Institute; Darcey Rodriguez, MLIS, is a liaison librarian at the Edward G. Miner Library; and Sally A. Norton, PhD, RN, FAAN, is a professor and associate dean for research in the School of Nursing, all at the University of Rochester Medical Center in New York. No financial relationships to disclose. Underhill-Blazey and Rodriguez contributed to the conceptualization and design and completed the data collection. Underhill-Blazey provided statistical support. Underhill-Blazey and Norton provided the analysis. All authors contributed to the manuscript preparation. Underhill-Blazey can be reached at meghan_blazey@urmc.rochester.edu with copy to ONFEditor@ons.org. (Submitted May 2021. Accepted October 22, 2021.)

