Finding the Optimal Exercise Dose While Living With Cancer-Related Fatigue: A Qualitative Study
Purpose: To gain insight into how survivors of breast cancer (SBCs) with cancer-related fatigue (CRF) self-monitor and manage exercise dose in the context of daily life, and how they identify an optimal exercise dose.
Participants & Setting: 11 SBCs with CRF who reported weekly exercise were recruited from a breast cancer center at a large urban hospital in the northeastern region of the United States.
Methodologic Approach: One-on-one semistructured interviews were conducted using a descriptive phenomenologic method. Inductive data analysis was performed within and across cases.
Findings: The following themes emerged: examining the impact of exercise, finding an optimal dose, and remaining flexible to sustain exercise. Participants used trial and error to explore exercise dose, examining the effects of varying doses on daily life. These effects had behavioral implications and resulted in a nonlinear process and the perception that an optimal exercise dose is dynamic within the context of daily life.
Implications for Nursing: Strategies to support SBCs with CRF to efficiently achieve optimal exercise doses with fewer setbacks may improve individuals’ ability to self-manage and mitigate CRF. This study’s findings provide practical approaches for nurses to encourage the initiation and adoption of exercise behaviors after treatment for breast cancer.
Jump to a section
Defined as a “sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” (Berger et al., 2015, p. 1020), cancer-related fatigue (CRF) is one of the most prevalent side effects experienced by survivors of breast cancer (SBCs) (Goedendorp et al., 2013; Jones et al., 2016; Thong et al., 2020). Compared to SBCs without CRF, SBCs with CRF report lower rates of employment (Ekenga et al., 2018), higher healthcare use (Heins et al., 2013), and a lower quality of life (Bower et al., 2014). In addition, CRF is associated with shorter recurrence-free and overall survival (Groenvold et al., 2007). For SBCs, systematic reviews and meta-analyses have established exercise as an effective intervention to reduce CRF (Juvet et al., 2017; Kessels et al., 2018; Meneses-Echávez et al., 2015a). Evidence further suggests there may be a dose–response relationship between exercise and CRF: Compared to less exercise, CRF reductions are greater when exercise sessions are more than 30 minutes and programs last longer than 12 weeks (Campbell et al., 2019; Meneses-Echávez et al., 2015b). Recently updated exercise guidelines for survivors of cancer also emphasize the influence of exercise intensity, recommending moderate- to vigorous-intensity exercise to mitigate CRF, emphasizing that low-intensity exercise is less effective (Brown et al., 2011; Campbell et al., 2019; Juvet et al., 2017; Meneses-Echávez et al., 2015a, 2015b). Taken together, the current evidence demonstrates that exercise dose is a critical moderator of the effectiveness of exercise to reduce CRF among SBCs.
However, SBCs with CRF often report exercise participation below recommended levels (Irwin et al., 2004; Schmidt et al., 2017; Wood et al., 2020), citing numerous barriers to exercise, including physical deconditioning, CRF itself, and the fear that exercise will worsen CRF (Blaney et al., 2010; Clifford et al., 2018). Such barriers, in addition to emerging evidence of increased fatigability (i.e., reduced endurance, greater perceived exertion, and decreased muscle force or power) among SBCs with CRF (Wood Magee et al., 2022), may limit this population’s ability to engage in exercise at recommended doses, thereby reducing the effectiveness of exercise to mitigate CRF. This may contribute to CRF persisting, which has been demonstrated in as many as one-third of SBCs (Abrahams et al., 2016; Goedendorp et al., 2013), resulting in poorer physical function, reduced postural control, and twice the rate of self-reported falls (Wechsler, Kneiss, et al., 2022; Wood et al., 2020). Given the consequences of persistent CRF on long-term health, it is critical to support SBCs to exercise at a safe and effective dose.
Although research has revealed barriers, facilitators, motivations, preferences, and perceived benefits of exercise among SBCs with CRF (Blaney et al., 2010, 2013; Clifford et al., 2018), the understanding of how this population self-monitors and manages their exercise dose is limited. A deeper understanding of how SBCs with CRF perceive and achieve an optimal (i.e., effective and sustainable) exercise dose would provide valuable insight for developing strategies that promote effective engagement in exercise to mitigate CRF. Therefore, the purpose of this study was to describe how SBCs with CRF self-monitor and manage exercise dose in the context of daily life, and how they perceive and identify an optimal exercise dose.
Methods
Study Design
As part of a larger sequential, mixed-methods study conducted from June 2021 to February 2022, a descriptive phenomenologic approach based on Husserl’s (1913/1983) philosophy was used to describe the lived experience of engaging in regular exercise among SBCs with persistent CRF (Wechsler, Fu, et al., 2022). Husserl’s (1913/1983) phenomenologic philosophy posits that every experience has intentionality and that an individual’s experience “can be exemplified for intuition in experiential data” (p. 11). Descriptive phenomenology has previously been used to explore how individuals living with chronic diseases (e.g., diabetes, lymphedema, systemic sclerosis) experience exercise and self-manage their disease (Arikan Dönmez et al., 2021; Lin et al., 2022; Pettersson et al., 2020; Yildirim Duman, 2021). Therefore, to explore how SBCs with CRF perceive and approach engaging in regular exercise, the authors of the current study chose a descriptive phenomenologic approach using one-on-one semistructured interviews. The current study sought to elucidate aspects of exercise dose within the lived experience of SBCs with persistent CRF who engage in regular exercise. All study procedures were approved by Massachusetts General Hospital’s institutional review board.
Participants and Recruitment
Participants were recruited from the Gillette Center for Women’s Cancer at Massachusetts General Hospital in Boston. Potential participants were identified by the principal investigator (S.W.) via an electronic health record review using the following inclusion criteria: female, aged 18–85 years, and having completed chemotherapy for stage I–III breast cancer at least 12 months prior to the time of EHR review (ongoing antihormone therapies permitted). The authors restricted participants’ age to target adult SBCs while minimizing the potential for age-related comorbidities that may confound the experience of fatigue or exercise participation. The presence of any of the following conditions, which typically include a component of fatigue, were used as exclusion criteria: precancer diagnosis of fibromyalgia, Post-Treatment Lyme Disease Syndrome, chronic fatigue syndrome, hypothyroidism without replacement therapy, anemia with hemoglobin levels less than 12 g/dl, or a positive screen for major depression or an anxiety disorder based on responses to the Patient Health Questionnaire–4 (Kroenke et al., 2009).
To explore the lived experience of engaging in regular exercise while living with CRF, participants were purposively sampled as key informants to participate in a qualitative interview if they met the additional following criteria: self-reported clinically significant CRF as indicated by a score of 50 or less on the SF-36® vitality subscale (Donovan et al., 2008; Ware, 1993) and any amount of weekly moderate-intensity exercise as self-reported via the Community Health Activities Model Program for Seniors questionnaire (Stewart et al., 2001).
Sample Size Calculation
A minimum sample size of six is recommended to explore the lived experience of a given phenomenon (for a phenomenologic study) (Gentles et al., 2015; Patton, 2020), although the adequacy of the sample size is ultimately determined by data saturation (i.e., similar data emerge or are repeated by multiple participants) (Patton, 2020; Sandelowski, 2000). Therefore, a priori, the authors of the current study estimated recruiting 6–20 interview participants, but the sample size was ultimately determined by data saturation.
Data Collection
The principal investigator (S.W.) conducted one-on-one, semistructured, audio-recorded interviews via a secure online platform. All participants consented to audio recordings. The research team developed an interview guide using a descriptive phenomenologic method (Bevan, 2014; Fu & Rosedale, 2009), and S.W. pilot tested this guide through an interview with a male survivor of nonbreast cancer who otherwise met inclusion criteria. The interview guide was developed to elicit discussion through five overarching questions about the participant’s experience with the following topics: (a) experience of CRF in daily life, (b) current exercise routine, (c) experience of CRF related to exercise, (d) experience immediately following exercise, and (e) confidence related to exercise. Probes were developed related to each overarching question and were iteratively revised as data collection progressed. As participants described experiences related to exercise dose, specific probes were added, including the following questions: (a) “How do you know that your current exercise routine is the optimal amount of exercise?” (b) “Other women have expressed a ‘threshold’ where if they push too hard or exercise too long, they may pay the price. Is this something you’ve experienced? If so, what strategies did you use to continue exercising, given this threshold?” and (c) “What amount and kind of exercise can you do in your daily life that will not result in worse fatigue?”
S.W. recorded field notes during and after each interview to aid in data analysis. All interviews were transcribed verbatim and proofread before analysis to ensure the accuracy of each participant’s responses.
Data Analysis
An iterative, multistep inductive process was used to examine the data, compare codes, challenge interpretations, and develop themes while maximizing the credibility and rigor of qualitative data analysis (Arikan Dönmez et al., 2021; Fu & Rosedale, 2009). The authors’ qualitative data analysis approach closely followed the methods detailed in the larger mixed-methods study (Wechsler, Fu, et al., 2022). Briefly, two authors (S.W. and M.R.F.) independently reviewed qualitative data and identified key quotations before meeting to develop a coding scheme, combine key quotations to formulate meaning, and develop themes. The full research team met to evaluate initial themes, develop major themes through active dialogue, and to reach a consensus on final themes that were supported by data using direct quotes from participants. To ensure representativeness of the themes (i.e., the degree to which the themes reflected views conveyed by many participants versus a select few), the authors returned to the data and examined the degree to which each theme and subtheme were apparent in data from each participant.
Results
Participants
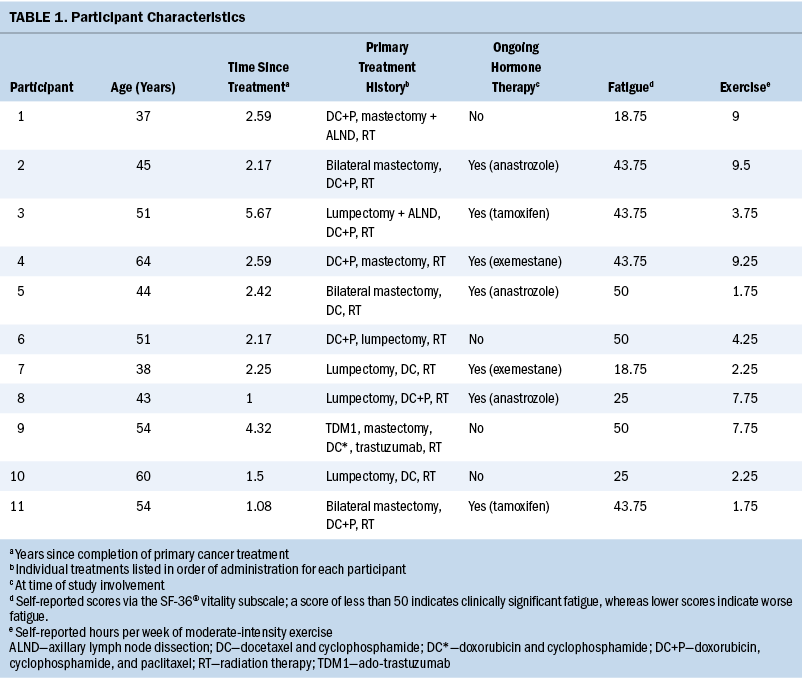
Of the 58 participants enrolled in the study, 19 endorsed clinically significant fatigue and weekly exercise, thus qualifying for participation in the qualitative interview. Data saturation was achieved with the ninth participant. Two additional participants were interviewed with no new data emerging, thus bringing the sample size to 11. Characteristics for the 11 participants are provided in Table 1. On average, participants were aged 49.2 years (SD = 8.6), were 2.5 years post-treatment (SD = 1.4), and scored 37.5 on the SF-36 vitality subscale (SD = 12.8). All participants met the minimum weekly recommended hours of moderate-intensity exercise per self-report with an average of 5.4 hours (SD = 3.3; median = 4.25; range = 1.75–9) (Campbell et al., 2019).
Essential Themes
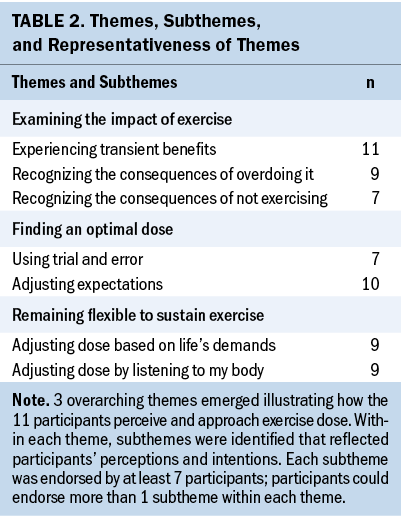
Three overarching themes emerged that illustrated how SBCs with CRF perceive and approach exercise dose: (a) examining the impact of exercise, (b) finding an optimal dose, and (c) remaining flexible to sustain exercise. Within each theme, subthemes were identified that reflected participants’ perceptions and intentions, where perceptions were interpreted as the meaning of the experience for each participant and intentions were viewed as participants’ conscious actions toward engaging in regular exercise. Each subtheme was endorsed by at least seven participants (see Table 2).
Theme 1: Examining the Impact of Exercise
As participants engaged in exercise after primary cancer treatment, they examined the impact of exercise on their fatigue and everyday lives.
Experiencing transient benefits: One woman (participant 4) who experienced transient benefits related to exercise stated, “I feel energized and happy . . . more alert, ready to face my day.” Another woman (participant 6) echoed the multidimensional benefits and said, “I usually have quite a bit of energy when I’m done. . . . My mood is definitely better; I think my brain functions clearer when I exercise.” Through these positive experiences, participants developed the perception that exercise is useful and can be used as a strategy to self-manage their CRF on a day-to-day basis.
Recognizing the consequences of overdoing it: Women also shared the experience of realizing that exercising at too great a dose (i.e., too intensely or for too long) resulted in negative consequences, such as worsened fatigue, disruptions to daily life, and fear of overexertion. In many cases, this was related to an attempt to resume pretreatment exercise intensity. When talking about overexerting herself, participant 10 said, “I would be wiped out for days. Not even just one day—two days, three days. I didn’t know I could even be that low.” Other participants related to the multiday effects of overdoing it, and participant 2 stated, “If I did too much . . . I’d be out the rest of the day and the next day.” Similar experiences led to participants fearing overexertion, to which participant 9 said, “I’m afraid of, you know, pushing myself too far because I feel like that’s bad for my health.” Participant 7 felt discouraged about rejoining an exercise class, fearing that trying to keep up with the intensity would be “embarrassing and/or lead to injury.”
Participant 11 had not experienced the consequences of exercising at too great a dose and stated, “I don’t know if I have the stamina to get [to an upper threshold of exercise] right now.” However, she still seemed to perceive consequences of exercising beyond a certain dose threshold and explained why walking was her preferred exercise. She said, “[Walking feels] like a safe thing. . . . It [feels] manageable because you can always slow your pace down or don’t go as far.”
Recognizing the consequences of not exercising: Conversely, participants shared the experience of realizing that exercising at too low of a dose also resulted in negative consequences, such as worsened fatigue, increased stiffness, and depressed mood. Participant 6 stated, “I started to learn by day 3 in this pattern of, ‘Don’t walk, don’t walk, don’t walk,’ and, ‘Ugh . . . I can’t get out of bed.’” Some participants noted a direct impact on their fatigue. Participant 1 said, “I do notice that if I don’t do anything . . . it’s [going to] worsen [my fatigue].” Others described physical and psychological consequences. Participant 2 stated, “I really can’t go more than two days without some type of movement. . . . My body gets stiffer. . . . It’s not good for my mind.” Participant 10 felt the same and said, “If you don’t exercise . . . you don’t feel good and then it’s just that awful cycle.” With this common experience, participants acknowledged that although exercise has transient benefits, exercising at an incorrect dose (too much or too little) can negatively affect their daily life.
Theme 2: Finding an Optimal Dose
Participants described a process of finding an optimal exercise dose through trial and error and by adjusting their expectations.
Using trial and error: Participant 2 shared her experience of when she began exercising after treatment and stated, “I would do a workout and I’d have to come home and take a nap, and that’s just how it started. . . . It was and still is a lot of trial and error.” She went on to emphasize the daily trial-and-error process and said, “To learn that fine line of what’s the good kind of push . . . every day I gauge, ‘Is this too much or is this just enough?’” Participant 1 similarly said, “[It is] a weird balance. . . . I have to do a little bit, but not too much.” She went on to illustrate the daily task of identifying an optimal exercise dose and stated, “Each day I’m trying to figure out the point where I’m not doing too much to overexert myself . . . but I also [want to] test out my limit. So, I try to test it out.”
Adjusting expectations: Participants described a dissonance between their post-treatment exercise capacity and their expectations. Participant 7 said, “It’s always about what your baseline was and what you feel like your new baseline is. . . . It’s really annoying and frustrating.” Women revealed a process of adjusting their expectations as an important part of finding an optimal exercise dose. Participant 9 said she felt the need to “not expect too much from myself and to be OK with not doing everything that I used to do.” Participant 1 faced a similar adjustment as she experienced physical limitations post-treatment and said, “I’m used to pushing myself and not listening to my body [when it says], ‘Hey, I can’t go that far.’” Looking toward her future exercise routine, she adjusted her expectations regarding her recovery, ongoing fatigue, and the need to adjust her exercise accordingly and said, “I don’t really anticipate [my fatigue] getting better. I just anticipate it as something that I have to adjust to in my life.” As participants found an optimal dose, they voiced the importance of allowing themselves grace and setting realistic expectations. Participant 2 said, “I just have to stay in the moment and be grateful for what my body can do in that moment . . . meeting my body where it’s at that day.”
Theme 3: Remaining Flexible to Sustain Exercise
Participants sustained regular exercise routines by continuously adjusting their dose based on life’s demands and by listening to their bodies.
Adjusting dose based on life’s demands: Participants budgeted their energy and adjusted their exercise dose to avoid interference with work and other activities. Participant 10 said, “You do have to be careful about how to organize your energy and how to make sure that you’re going to have enough for the things that you really, really have to do.” Participant 2 reduced her exercise intensity and said, “I don’t go to that next gear because I know it’s probably too much stress on my body, and then I’m going to be unable to do everything else I need to do in a day.” Similarly, participant 4 said she adjusted her walking distance “on days that I had to work or I had to do something. I would know that I couldn’t do above two and a half miles, or I was no good.”
Adjusting dose by listening to my body: Participants also learned to recognize physical sensations as indications to adjust their exercise dose. Participant 2 recognized neuropathic symptoms as a dose-limiting factor and said, “There’s just this tipping point. . . . The sweet spot for me is [walking] about four miles. If I do more than that, then I get the neuropathy in my feet.” Participant 8 also relied on her body’s signals and said, “You push your body. . . . Sometimes I feel nauseous. . . . I hit a point where I’m just like, ‘Enough is enough.’ . . . My body tells me.” Participants also progressed their exercise dose based on how they felt. Participant 3 stated, “I feel like more exercise feels better for me and sustains that little bit of a high for longer.” Participant 6 associated benefits with increased exercise frequency and said, “Every morning I walked . . . that’s when I realized I was feeling better. . . . Frequency makes a big difference.” Similarly, Participant 11 was motivated to increase her exercise frequency based on positive experiences after exercise and said, “You feel motivated to do it again ’cause it does feel good.”
Discussion
Despite varying exercise preferences, levels of participation, and severity of CRF, participants shared common processes and pitfalls related to finding an optimal exercise dose after cancer treatment. The trial-and-error process described by participants appeared slow and inefficient as women often oscillated between upper and lower thresholds of exercise. In addition, women encountered discouraging setbacks through this process when their exercise dose oscillated beyond these thresholds, resulting in worsened fatigue, fear of overexertion, and difficulty maintaining life roles. Discouragement and fear may result in reduced exercise adherence or dose (Nijs et al., 2004; Saanijoki et al., 2015; Silver et al., 2002), limiting the effectiveness of exercise to reduce CRF. This may have been reflected in this sample, because all participants continued to experience clinically significant CRF despite weekly exercise. These findings suggest a need to consider the behavioral implications of the processes described by the participants and to identify strategies to support SBCs with CRF to achieve optimal exercise doses more efficiently and with fewer setbacks.
Participants described challenges identifying an initial exercise dose after cancer treatment. This lack of knowledge regarding a safe and effective exercise type and exercise intensity is a known barrier to exercise initiation among survivors of cancer (Clifford et al., 2018). Although most sources recommend that survivors should initiate exercise at a low intensity and progress slowly (Avancini et al., 2020; Schwartz et al., 2017), current guidelines lack specificity regarding an initial dose for survivors with CRF. Given the challenges described by the participants in the current study and the potential consequences of initiating exercise at too high or too low an intensity, completing fitness and functional assessments prior to exercise initiation—a practice that is currently recommended for only older adult survivors of cancer (Campbell et al., 2019)—may be useful for SBCs with CRF to identify an appropriate initial dose. At a minimum, clinicians should provide guidance regarding initial exercise dose by individualizing exercise guidelines in the context of a patient’s pretreatment fitness level, current physical function, and other relevant cultural and environmental factors (Schwartz et al., 2017). An individualized initial exercise dose and education on how to subsequently adapt dose (increase or decrease intensity, duration, and/or frequency) based on individuals’ exercise performance over time may hold promise for enhancing exercise behaviors in this population. Individualized and adaptive interventions acknowledge that health behavior change is a nonlinear process (Heino et al., 2021; Resnicow & Vaughan, 2006) and have been shown to result in sustained changes in physical activity among inactive healthy individuals and inactive individuals who are obese (Adams et al., 2017; Poirier et al., 2016).
In the absence of guidance regarding how to adjust dose over time, participants described learning to rely on their body’s responses to adjust exercise dose. Learning this strategy was a product of trial and error and, as a result, took time. A greater ability to self-monitor physiologic, cognitive, and emotional responses to exercise may allow SBCs with CRF to increase or decrease their dose more accurately, leading to fewer oscillations beyond upper or lower thresholds, thereby reducing setbacks. Fewer setbacks and more appropriately dosed exercise, leading to the benefits (e.g., improved energy and mood) described by the current study’s participants, may reinforce exercise behaviors (Bandura, 1976). Such positive reinforcement is an integral component of several well-known theoretical approaches to behavior change (e.g., Transtheoretical Model [Prochaska, 2020], social cognitive theory [Stacey et al., 2015]), which have been shown to enhance the effectiveness of interventions aimed at increasing physical activity among survivors of cancer (Berkman & Gilchrist, 2018). Another critical component of these behavioral theories is exercise self-efficacy, which is a known facilitator of exercise participation among SBCs (Awick et al., 2017; Wechsler, Fu, et al., 2022). Evidence demonstrates that self-monitoring interventions can enhance physical activity among survivors of cancer (Ormel et al., 2018; Singh et al., 2022) and may concurrently enhance exercise self-efficacy (Picha & Howell, 2018). Clinicians can teach self-monitoring skills using a variety of approaches (e.g., activity tracking devices, exercise logs, self-monitoring of vital signs/perceived exertion), which may lead to a more gradual and linear increase in exercise dose and a more timely achievement of more optimal levels of exercise to reduce CRF. Figure 1 depicts a conceptual model comparing the trial-and-error nonlinear process of exercising described by participants and the authors’ hypothesized more efficient process to achieve an optimal exercise dose.
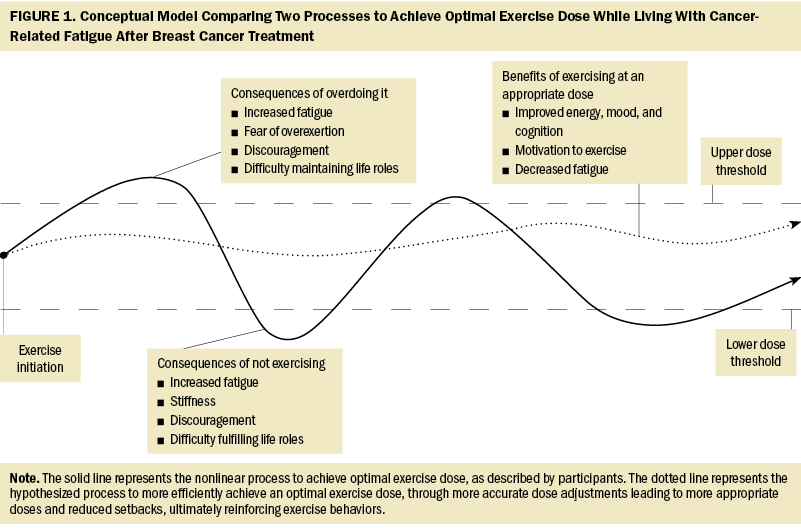
Finally, SBCs with CRF in this sample perceived exercise dose as dynamic, and they continuously adjusted exercise intensity, duration, and frequency to maintain their life roles. This is consistent with prior evidence showing that survivors with and without CRF prefer flexible exercise routines (Blaney et al., 2013; Craike et al., 2017; Whitehead & Lavelle, 2009), but is divergent from current guidelines that recommend a static dose of three 30-minute bouts of aerobic exercise per week to reduce CRF (Campbell et al., 2019). The current study’s participants described a need to consider numerous factors to determine the optimal dose of exercise for any given day, suggesting adhering to a static dose of exercise may be challenging. An adaptive exercise intervention may also address this challenge by frequently adjusting exercise dose in response to fluctuations in exercise behaviors in the context of daily life. In addition, exercise interventions that include problem-solving and action-planning behavior change techniques have been shown to be feasible and acceptable strategies to foster functional recovery after breast cancer (Hegel et al., 2011; Lyons et al., 2012) and may enhance individuals’ ability to effectively plan exercise and manage energy resources. These techniques have been demonstrated to be effective in increasing physical activity among adults (Gardner et al., 2016; Senkowski et al., 2019) and may ultimately lead to greater exercise adherence and improved self-management of CRF among SBCs.
Physical activity guidelines from the American Cancer Society suggest that SBCs should perform any amount of physical activity they can to mitigate the consequences of inactivity (Rock et al., 2022), suggesting a more flexible approach to exercise dose may be beneficial, although evidence of this strategy to reduce CRF is limited. Given evidence that flexible prescriptions of short exercise bouts can improve exercise adherence and physiological outcomes in healthy and chronically ill populations (DeBusk et al., 1990; Jakicic et al., 1995; Schwartz et al., 2001), future research should assess the acceptability and efficacy of more flexible and adaptive exercise doses to reduce CRF among SBCs.
Limitations
Although the goal of a qualitative study is to gain a deeper understanding of a phenomenon, generalizability is not intended, and these results should be interpreted in this context. These qualitative data reflect the experiences of 11 female SBCs with CRF living in the United States. It should be noted that although each subtheme was endorsed by at least 7 of the 11 participants, this does not indicate that the remaining participants disagreed or provided counter perspectives to these concepts. Still, the essence of the experiences drawn from these 11 women does not necessarily represent how all SBCs perceive and approach exercise. In addition, the participants, sampled because of their weekly exercise engagement, had found ways to achieve a sustainable exercise dose despite the setbacks they encountered. Therefore, this study does not represent the experience of SBCs with CRF who attempt but do not sustain exercise participation. The perspective of exercise-nonadherent individuals may be valuable to provide additional insight into how SBCs with CRF approach exercise dose. Nonetheless, to the authors’ knowledge, this study was the first to explore how SBCs with CRF perceive and manage exercise dose in the context of daily life. Future studies focused on the concept of exercise dose among more diverse (e.g., race, cancer diagnosis, physical activity level) survivors of cancer with CRF may further elucidate important aspects of this experience and may improve generalizability.
Implications for Nursing
Oncology nurses have long played a critical role in symptom measurement and management during and after cancer treatment (White et al., 2019; Young et al., 2020). Given the efficacy of exercise to decrease symptom burden in SBCs and considering the frequency with which oncology nurses are in contact with survivors, nurses are in a unique position to provide guidance regarding the positive effects of exercise in reducing CRF (Turner et al., 2018). SBCs with CRF have described oncology nurses as important members of their support systems related to engaging in exercise after cancer treatment (Wechsler, Fu, et al., 2022). In addition, research has demonstrated that a nurse-led physical activity coaching program can increase physical activity and decrease fatigue (Forner et al., 2021). However, nurses have described feeling unsure about what dose to recommend as a barrier to promoting physical activity (Karvinen et al., 2012). Although future studies are needed to develop and test nurse-led interventions to support SBCs with CRF to achieve an optimal exercise dose, this study provides insight to assist nurses in discussing and encouraging initiation and adoption of exercise behaviors after treatment for breast cancer.
Aligned with the recently published CRF Self-Management Support Practice Framework (Agbejule et al., 2023), oncology nurses should consider clinical and behavioral information when advising SBCs with CRF regarding an initial exercise dose. Nurses should provide support and education about potential changes in exercise capacity post-treatment compared to pretreatment. Nurses should also consider educating and empowering survivors to self-monitor their responses (e.g., physical, emotional, cognitive) during and after exercise, and to make small adjustments to exercise dose to avoid overdoing or underdoing it. Providing encouragement and coaching to gradually increase exercise dose in the context of daily life may reinforce exercise behaviors and support SBCs with CRF to achieve higher doses of exercise more efficiently.

Conclusion
In summary, this study’s findings provide insight into how SBCs with CRF self-manage exercise dose and the behavioral implications that may inhibit or facilitate exercise adherence and progression to an optimal exercise dose. Future research is needed to develop interventions to support SBCs with CRF to initiate and engage in exercise at doses that will reinforce rather than discourage exercise participation and lead to efficient achievement of effective exercise doses to reduce CRF. Strategies may include accurately identifying individualized initial exercise dose after cancer treatment, teaching exercise self-monitoring, and promoting problem-solving and action planning around exercise engagement to optimize self-management of energy resources and incorporation of exercise into the context of daily life.

About the Authors
Stephen Wechsler, PT, DPT, PhD, NCS, is a postdoctoral fellow in the Department of Occupational Therapy at the MGH Institute of Health Professions in Charlestown, MA; Mei Rosemary Fu, PhD, RN, FAAN, is the Dorothy and Dale Thompson/Missouri Endowed Professor in Nursing and the associate dean for research in the School of Nursing and Health Studies University of Missouri—Kansas City; Lisa Wood Magee, PhD, RN, FAAN, is a professor in the William F. Connell School of Nursing at Boston College in Chestnut Hill, MA; and Kathleen D. Lyons, ScD, OTR/L, is a professor in the Department of Occupational Therapy at the MGH Institute of Health Professions. This research was funded, in part, by a National Institute on Aging grant (R21AG055149) and a postdoctoral research grant from Boston College. Wechsler, Fu, and Wood Magee contributed to the conceptualization and design. Wechsler and Wood Magee completed the data collection and provided statistical support. All authors provided the analysis and contributed to the manuscript preparation. Wechsler can be reached at swechsler@mghihp.edu, with copy to ONFEditor@ons.org. (Submitted March 2023. Accepted April 20, 2023.)
References
Abrahams, H.J.G., Gielissen, M.F.M., Schmits, I.C., Verhagen, C.A.H.H.V.M., Rovers, M.M., & Knoop, H. (2016). Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12 327 breast cancer survivors. Annals of Oncology, 27(6), 965–974. https://doi.org/10.1093/annonc/mdw099
Adams, M.A., Hurley, J.C., Todd, M., Bhuiyan, N., Jarrett, C.L., Tucker, W.J., . . . Angadi, S.S. (2017). Adaptive goal setting and financial incentives: A 2 × 2 factorial randomized controlled trial to increase adults’ physical activity. BMC Public Health, 17(1), 286. https://doi.org/10.1186/s12889-017-4197-8
Agbejule, O.A., Hart, N.H., Ekberg, S., & Chan, R.J. (2023). Development of a self-management support practice framework for addressing cancer-related fatigue: A modified Delphi study. Journal of Cancer Survivorship. https://doi.org/10.1007/s11764-023-01348-7
Arikan Dönmez, A., Kuru Alici, N., & Borman, P. (2021). Lived experiences for supportive care needs of women with breast cancer-related lymphedema: A phenomenological study. Clinical Nursing Research, 30(6), 799–808. https://doi.org/10.1177/1054773820958115
Avancini, A., Pala, V., Trestini, I., Tregnago, D., Mariani, L., Sieri, S., . . . Lanza, M. (2020). Exercise levels and preferences in cancer patients: A cross-sectional study. International Journal of Environmental Research and Public Health, 17(15), 5351. https://doi.org/10.3390/ijerph17155351
Awick, E.A., Phillips, S.M., Lloyd, G.R., & McAuley, E. (2017). Physical activity, self-efficacy and self-esteem in breast cancer survivors: A panel model. Psycho-Oncology, 26(10), 1625–1631. https://doi.org/10.1002/pon.4180
Bandura, A. (1976). Self-reinforcement: Theoretical and methodological considerations. Behaviorism, 4(2), 135–155. http://www.jstor.org/stable/27758862
Berger, A.M., Mooney, K., Alvarez-Perez, A., Breitbart, W.S., Carpenter, K.M., Cella, D., . . . Smith, C. (2015). Cancer-related fatigue, version 2.2015. Journal of the National Comprehensive Cancer Network, 13(8), 1012–1039. https://doi.org/10.6004/jnccn.2015.0122
Berkman, A.M., & Gilchrist, S.C. (2018). Behavioral change strategies to improve physical activity after cancer treatment. Rehabilitation Oncology, 36(3), 152–160. https://doi.org/10.1097/01.Reo.0000000000000112
Bevan, M.T. (2014). A method of phenomenological interviewing. Qualitative Health Research, 24(1), 136–144. https://doi.org/10.1177/1049732313519710
Blaney, J., Lowe-Strong, A., Rankin, J., Campbell, A., Allen, J., & Gracey, J. (2010). The cancer rehabilitation journey: Barriers to and facilitators of exercise among patients with cancer-related fatigue. Physical Therapy, 90(8), 1135–1147.
Blaney, J.M., Lowe-Strong, A., Rankin-Watt, J., Campbell, A., & Gracey, J.H. (2013). Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: A questionnaire–survey. Psycho-Oncology, 22(1), 186–194. https://doi.org/10.1002/pon.2072
Bower, J.E., Bak, K., Berger, A., Breitbart, W., Escalante, C.P., Ganz, P.A., . . . Jacobsen, P.B. (2014). Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology clinical practice guideline adaptation. Journal of Clinical Oncology, 32(17), 1840–1850.
Brown, J.C., Huedo-Medina, T.B., Pescatello, L.S., Pescatello, S.M., Ferrer, R.A., & Johnson, B.T. (2011). Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: A meta-analysis. Cancer Epidemiology, Biomarkers and Prevention, 20(1), 123–133. https://doi.org/10.1158/1055-9965.Epi-10-0988
Campbell, K.L., Winters-Stone, K.M., Wiskemann, J., May, A.M., Schwartz, A.L., Courneya, K.S., . . . Schmitz, K.H. (2019). Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Medicine and Science in Sports and Exercise, 51(11), 2375–2390. https://doi.org/10.1249/MSS.0000000000002116
Clifford, B.K., Mizrahi, D., Sandler, C.X., Barry, B.K., Simar, D., Wakefield, C.E., & Goldstein, D. (2018). Barriers and facilitators of exercise experienced by cancer survivors: A mixed methods systematic review. Supportive Care in Cancer, 26(3), 685–700. https://doi.org/10.1007/s00520-017-3964-5
Craike, M., Hose, K., Courneya, K.S., Harrison, S.J., & Livingston, P.M. (2017). Physical activity preferences for people living with multiple myeloma: A qualitative study. Cancer Nursing, 40(5), E1–E8. https://doi.org/10.1097/ncc.0000000000000425
DeBusk, R.F., Stenestrand, U., Sheehan, M., & Haskell, W.L. (1990). Training effects of long versus short bouts of exercise in healthy subjects. American Journal of Cardiology, 65(15), 1010–1013. https://doi.org/10.1016/0002-9149(90)91005-q
Donovan, K.A., Jacobsen, P.B., Small, B.J., Munster, P.N., & Andrykowski, M.A. (2008). Identifying clinically meaningful fatigue with the fatigue symptom inventory. Journal of Pain and Symptom Management, 36(5), 480–487. https://doi.org/10.1016/j.jpainsymman.2007.11.013
Ekenga, C.C., Pérez, M., Margenthaler, J.A., & Jeffe, D.B. (2018). Early-stage breast cancer and employment participation after 2 years of follow-up: A comparison with age-matched controls. Cancer, 124(9), 2026–2035.
Forner, J.K., Doughty, A., Dalstrom, M., Messer, B.L., & Lizer, S.K. (2021). Quality of life: A nurse-led physical activity coaching program to improve the quality of life of patients with cancer during the COVID-19 pandemic. Clinical Journal of Oncology Nursing, 25(5), 571–577. https://doi.org/10.1188/21.CJON.571-577
Fu, M.R., & Rosedale, M. (2009). Breast cancer survivors’ experiences of lymphedema-related symptoms. Journal of Pain and Symptom Management, 38(6), 849–859. https://doi.org/10.1016/j.jpainsymman.2009.04.030
Gardner, B., Smith, L., Lorencatto, F., Hamer, M., & Biddle, S.J.H. (2016). How to reduce sitting time? A review of behaviour change strategies used in sedentary behaviour reduction interventions among adults. Health Psychology Review, 10(1), 89–112. https://doi.org/10.1080/17437199.2015.1082146
Gentles, S.J., Charles, C., Ploeg, J., & McKibbon, K.A. (2015). Sampling in qualitative research: Insights from an overview of the methods literature. Qualitative Report, 20(11), 1772–1789.
Goedendorp, M.M., Gielissen, M.F.M., Verhagen, C.A.H.H.V.M., & Bleijenberg, G. (2013). Development of fatigue in cancer survivors: A prospective follow-up study from diagnosis into the year after treatment. Journal of Pain and Symptom Management, 45(2), 213–222. https://doi.org/10.1016/j.jpainsymman.2012.02.009
Groenvold, M., Petersen, M.A., Idler, E., Bjorner, J.B., Fayers, P.M., & Mouridsen, H.T. (2007). Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Research and Treatment, 105(2), 209–219. https://doi.org/10.1007/s10549-006-9447-x
Hegel, M.T., Lyons, K.D., Hull, J.G., Kaufman, P., Urquhart, L., Li, Z., & Ahles, T.A. (2011). Feasibility study of a randomized controlled trial of a telephone-delivered problem-solving—Occupational therapy intervention to reduce participation restrictions in rural breast cancer survivors undergoing chemotherapy. Psycho-Oncology, 20(10), 1092–1101. https://doi.org/10.1002/pon.1830
Heino, M.T.J., Knittle, K., Noone, C., Hasselman, F., & Hankonen, N. (2021). Studying behaviour change mechanisms under complexity. Behavioral Sciences, 11(5), 77. https://doi.org/10.3390/bs11050077
Heins, M.J., Korevaar, J.C., Rijken, P.M., & Schellevis, F.G. (2013). For which health problems do cancer survivors visit their general practitioner? European Journal of Cancer, 49(1), 211–218. https://doi.org/10.1016/j.ejca.2012.07.011
Husserl, E. (1983). Ideas pertaining to a pure phenomenology and to a phenomenological philosophy. First book: General introduction to a pure phenomenology (F. Kersten, Trans.) (Vol. 2). Martinus Nijhoff Publishers. https://www.finophd.eu/wp-content/uploads/2018/01/Husserl-Ideas-First-B… (Original work published 1913)
Irwin, M.L., McTiernan, A., Bernstein, L., Gilliland, F.D., Baumgartner, R., Baumgartner, K., & Ballard-Barbash, R. (2004). Physical activity levels among breast cancer survivors. Medicine and Science in Sports and Exercise, 36(9), 1484–1491. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3000611/pdf/nihms250943.pdf
Jakicic, J.M., Wing, R.R., Butler, B.A., & Robertson, R.J. (1995). Prescribing exercise in multiple short bouts versus one continuous bout: Effects on adherence, cardiorespiratory fitness, and weight loss in overweight women. International Journal of Obesity and Related Metabolic Disorders, 19(12), 893–901.
Jones, J.M., Olson, K., Catton, P., Catton, C.N., Fleshner, N.E., Krzyzanowska, M.K., . . . Howell, D. (2016). Cancer-related fatigue and associated disability in post-treatment cancer survivors. Journal of Cancer Survivorship, 10(1), 51–61.
Juvet, L.K., Thune, I., Elvsaas, I.K.Ø., Fors, E.A., Lundgren, S., Bertheussen, G., . . . Oldervoll, L.M. (2017). The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis. Breast, 33, 166–177. https://doi.org/10.1016/j.breast.2017.04.003
Karvinen, K.H., McGourty, S., Parent, T., & Walker, P.R. (2012). Physical activity promotion among oncology nurses. Cancer Nursing, 35(3), E41–E48. https://doi.org/10.1097/NCC.0b013e31822d9081
Kessels, E., Husson, O., & van der Feltz-Cornelis, C.M. (2018). The effect of exercise on cancer-related fatigue in cancer survivors: A systematic review and meta-analysis. Neuropsychiatric Disease and Treatment, 14, 479–494. https://doi.org/10.2147/NDT.S150464
Kroenke, K., Spitzer, R.L., Williams, J.B.W., & Löwe, B. (2009). An ultra-brief screening scale for anxiety and depression: The PHQ-4. Psychosomatics, 50(6), 613–621. https://doi.org/10.1176/appi.psy.50.6.613
Lin, T.-R., Huang, X.-Y., & Hwu, C.-M. (2022). Exercise experiences of older adults with diabetes and sarcopenia: A phenomenological study. Clinical Nursing Research, 31(2), 292–300. https://doi.org/10.1177/10547738211039381
Lyons, K.D., Erickson, K.S., & Hegel, M.T. (2012). Problem-solving strategies of women undergoing chemotherapy for breast cancer. Canadian Journal of Occupational Therapy, 79(1), 33–40. https://doi.org/10.2182/cjot.2012.79.1.5
Meneses-Echávez, J.F., González-Jiménez, E., & Ramírez-Vélez, R. (2015a). Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: A systematic review and meta-analysis. BMC Cancer, 15, 77. https://doi.org/10.1186/s12885-015-1069-4
Meneses-Echávez, J.F., González-Jiménez, E., & Ramírez-Vélez, R. (2015b). Supervised exercise reduces cancer-related fatigue: A systematic review. Journal of Physiotherapy, 61(1), 3–9. https://doi.org/10.1016/j.jphys.2014.08.019
Nijs, J., De Meirleir, K., & Duquet, W. (2004). Kinesiophobia in chronic fatigue syndrome: Assessment and associations with disability. Archives of Physical Medicine and Rehabilitation, 85(10), 1586–1592. https://doi.org/10.1016/j.apmr.2003.12.033
Ormel, H.L., van der Schoot, G.G.F., Westerink, N.-D.L., Sluiter, W.J., Gietema, J.A., & Walenkamp, A.M.E. (2018). Self-monitoring physical activity with a smartphone application in cancer patients: A randomized feasibility study (SMART-trial). Supportive Care in Cancer, 26(11), 3915–3923.
Patton, C.M. (2020). Phenomenology for the holistic nurse researcher: Underpinnings of descriptive and interpretive traditions. Journal of Holistic Nursing, 38(3), 278–286.
Pettersson, H., Nordin, A., Svenungsson, E., Alexanderson, H., & Boström, C. (2020). Experiences of physical activity and exercise in individuals with systemic sclerosis: A qualitative study. Musculoskeletal Care, 18(2), 150–160. https://doi.org/10.1002/msc.1447
Picha, K.J., & Howell, D.M. (2018). A model to increase rehabilitation adherence to home exercise programmes in patients with varying levels of self-efficacy. Musculoskeletal Care, 16(1), 233–237. https://doi.org/10.1002/msc.1194
Poirier, J., Bennett, W.L., Jerome, G.J., Shah, N.G., Lazo, M., Yeh, H.-C., . . . Cobb, N.K. (2016). Effectiveness of an activity tracker- and internet-based adaptive walking program for adults: A randomized controlled trial. Journal of Medical Internet Research, 18(2), e34. https://doi.org/10.2196/jmir.5295
Prochaska, J.O. (2020). Transtheoretical model of behavior change. In M.D. Gellman (Ed.), Encyclopedia of behavioral medicine (pp. 2266–2270). Springer. https://doi.org/10.1007/978-3-030-39903-0_70
Resnicow, K., & Vaughan, R. (2006). A chaotic view of behavior change: A quantum leap for health promotion. International Journal of Behavioral Nutrition and Physical Activity, 3, 25. https://doi.org/10.1186/1479-5868-3-25
Rock, C.L., Thomson, C.A., Sullivan, K.R., Howe, C.L., Kushi, L.H., Caan, B.J., . . . McCullough, M.L. (2022). American Cancer Society nutrition and physical activity guideline for cancer survivors. CA: A Cancer Journal for Clinicians, 72(3), 230–262. https://doi.org/10.3322/caac.21719
Saanijoki, T., Nummenmaa, L., Eskelinen, J.-J., Savolainen, A.M., Vahlberg, T., Kalliokoski, K.K., & Hannukainen, J.C. (2015). Affective responses to repeated sessions of high-intensity interval training. Medicine and Science in Sports and Exercise, 47(12), 2604–2611. https://doi.org/10.1249/mss.0000000000000721
Sandelowski, M. (2000). Combining qualitative and quantitative sampling, data collection, and analysis techniques in mixed-method studies. Research in Nursing and Health, 23(3), 246–255.
Schmidt, M.E., Wiskemann, J., Ulrich, C.M., Schneeweiss, A., & Steindorf, K. (2017). Self-reported physical activity behavior of breast cancer survivors during and after adjuvant therapy: 12 months follow-up of two randomized exercise intervention trials. Acta Oncologica, 56(4), 618–627.
Schwartz, A.L., de Heer, H.D., & Bea, J.W. (2017). Initiating exercise interventions to promote wellness in cancer patients and survivors. Oncology, 31(10), 711–717.
Schwartz, A.L., Mori, M., Gao, R., Nail, L.M., & King, M.E. (2001). Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Medicine and Science in Sports and Exercise, 33(5), 718–723.
Senkowski, V., Gannon, C., & Branscum, P. (2019). Behavior change techniques used in theory of planned behavior physical activity interventions among older adults: A systematic review. Journal of Aging and Physical Activity, 27(5), 746–754.
Silver, A., Haeney, M., Vijayadurai, P., Wilks, D., Pattrick, M., & Main, C.J. (2002). The role of fear of physical movement and activity in chronic fatigue syndrome. Journal of Psychosomatic Research, 52(6), 485–493. https://doi.org/10.1016/S0022-3999(01)00298-7
Singh, B., Zopf, E.M., & Howden, E.J. (2022). Effect and feasibility of wearable physical activity trackers and pedometers for increasing physical activity and improving health outcomes in cancer survivors: A systematic review and meta-analysis. Journal of Sport and Health Science, 11(2), 184–193.
Stacey, F.G., James, E.L., Chapman, K., Courneya, K.S., & Lubans, D.R. (2015). A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. Journal of Cancer Survivorship, 9(2), 305–338.
Stewart, A.L., Mills, K.M., King, A.C., Haskell, W.L., Gillis, D., & Ritter, P.L. (2001). CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Medicine and Science in Sports and Exercise, 33(7), 1126–1141.
Thong, M.S.Y., van Noorden, C.J.F., Steindorf, K., & Arndt, V. (2020). Cancer-related fatigue: Causes and current treatment options. Current Treatment Options in Oncology, 21(2), 17. https://doi.org/10.1007/s11864-020-0707-5
Turner, R.R., Steed, L., Quirk, H., Greasley, R.U., Saxton, J.M., Taylor, S.J., . . . Bourke, L. (2018). Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database of Systematic Reviews, 9(9), CD010192. https://doi.org/10.1002/14651858.CD010192.pub3
Ware, J.E., Jr. (1993). SF-36 health survey: Manual and interpretation guide. The Health Institute, New England Medical Center. https://www.researchgate.net/profile/John-Ware-6/publication/313050850_…
Wechsler, S., Fu, M.R., Lyons, K., Wood, K.C., & Wood Magee, L.J. (2022). The role of exercise self-efficacy in exercise participation among women with persistent fatigue after breast cancer: A mixed-methods study. Physical Therapy, 103(1), pzac143.
Wechsler, S., Kneiss, J., Adams, B., & Wood Magee, L.J. (2022). Persistent cancer-related fatigue after breast cancer treatment predicts postural sway and post-exertional changes in sit-to-stand strategy. Rehabilitation Oncology, 40(4), 162–171. https://doi.org/10.1097/01.Reo.0000000000000308
White, L.L., Cohen, M.Z., Berger, A.M., Kupzyk, K.A., & Bierman, P.J. (2019). Self-efficacy for management of symptoms and symptom distress in adults with cancer: An integrative review. Oncology Nursing Forum, 46(1), 113–128. https://doi.org/10.1188/19.ONF.113-128
Whitehead, S., & Lavelle, K. (2009). Older breast cancer survivors’ views and preferences for physical activity. Qualitative Health Research, 19(7), 894–906. https://doi.org/10.1177/1049732309337523
Wood, L.J., Winters-Stone, K.M., Kneiss, J.A., Fox, A.B., & Walker, R.K. (2020). Women with clinically significant fatigue after breast cancer treatment report increased falls and perform poorly on objective measures of physical fitness and function. Rehabilitation Oncology, 38(2), 92–99.
Wood Magee, L.J., Kneiss, J., Wechsler, S., Singh, A.B., Fox, A.B., Peppercorn, J., & Pirl, W.F. (2022). Increased fatigability in women with persistent cancer-related fatigue after breast cancer treatment: A pilot study. Rehabilitation Oncology, 40(3), 135–144. https://doi.org/10.1097/01.Reo.0000000000000305
Yildirim Duman, J.G. (2021). Self-management of chronic diseases: A descriptive phenomenological study. Social Work in Public Health, 36(2), 300–310.
Young, A.M., Charalambous, A., Owen, R.I., Njodzeka, B., Oldenmenger, W.H., Alqudimat, M.R., & So, W.K.W. (2020). Essential oncology nursing care along the cancer continuum. Lancet Oncology, 21(12), e555–e563.



