Stress Exposures Contribute to Worse Joint Morning and Evening Fatigue Profiles in Patients With Cancer During Chemotherapy
Objectives: To evaluate differences among stress, resilience, and coping strategies related to morning and evening fatigue profiles (both low, low morning and moderate evening, both moderate, and both high).
Sample & Setting: Data were collected from 1,334 adult patients with cancer receiving chemotherapy.
Methods & Variables: Morning and evening fatigue severity were rated over two cycles of chemotherapy using the Lee Fatigue Scale. Latent profile analysis was used to identify patient subgroups with distinct joint morning and evening profiles. Data were collected on global, cancer-specific, and cumulative life stress; resilience; and coping strategies. Differences among the latent classes were evaluated using parametric and nonparametric tests.
Results: Compared to the other three classes, the both high class reported the highest stress scores, highest occurrence of and effects from a variety of stressful life events, lowest resilience scores, and higher use of disengagement coping strategies. The both high class met the criteria for subsyndromal post-traumatic stress disorder.
Implications for Nursing: When patients report high levels of fatigue, detailed assessments of stress are warranted to provide tailored interventions.
Jump to a section
Fatigue is a common symptom that limits the daily activities and quality of life of patients with cancer (National Comprehensive Cancer Network, 2023). Although most studies evaluate average fatigue severity (i.e., fatigue score without consideration of diurnal variations), a growing body of evidence suggests that diurnal variations in fatigue severity warrant additional investigation because different types of interventions may be warranted (Dhruva et al., 2013; Dimsdale et al., 2007; Pietrowsky & Lahl, 2008). In the authors’ previous studies of morning and evening fatigue as single symptoms, common and distinct risk factors (Wright, Cooper, et al., 2017; Wright et al., 2015, 2019) and underlying mechanisms (Kober et al., 2023; Wright, Hammer, et al., 2017) were identified.
More recently, to gain additional insights into patients who were at increased risk for high levels of both morning and evening fatigue, the authors used latent profile analysis to identify the following four classes of patients with distinct joint morning and evening fatigue profiles: both low (24%), low morning and moderate evening (26%), both moderate (39%), and both high (12%) (Wright et al., 2023). Risk factors associated with the worst severity profiles included younger age, decreased likelihood of being married or partnered, increased likelihood of living alone, and having a higher comorbidity burden and a lower functional status. In addition, patients with the worst severity profiles reported higher levels of anxiety, depression, sleep disturbance, and pain, as well as lower quality-of-life scores. The current article extends these findings by evaluating for differences in stress, resilience, and coping among the four distinct joint morning and evening fatigue profiles.
Background
Fatigue and Stress
In healthy individuals, fatigue is an adaptive response to acute stress that conserves energy and maintains homeostasis (Kop & Kupper, 2016). Intense, persistent, and/or cumulative life stress activates the autonomic nervous system and can overwhelm the regulatory feedback loop of the hypothalamic–pituitary–adrenal axis. This process results in a loss of homeostasis and an increase in allostatic load that leads to higher levels of physical fatigue (Shields & Slavich, 2017; Starr et al., 2019; Thorsteinsson et al., 2019). The chronicity and intensity of the stress, types of stressors, coping abilities, and environmental factors moderate an individual’s responses to stress (Galatzer-Levy et al., 2018).
Previous studies of patients with cancer found that higher levels of average fatigue were associated with higher levels of global stress (Higgins et al., 2008; Ho et al., 2015; Reinertsen et al., 2017; Sakamoto et al., 2017; Yeh, 2021), cancer-specific stress (Bower et al., 2021; Cohen et al., 2019; Fagundes et al., 2012; Higgins et al., 2008; Von Ah et al., 2008; Yee et al., 2017), and cumulative life stress (Bean et al., 2021; Bower et al., 2014, 2019, 2021; Fagundes et al., 2012). However, most of these studies included only women with breast cancer (Bean et al., 2021; Bower et al., 2014, 2019, 2021; Cohen et al., 2019; Fagundes et al., 2012; Higgins et al., 2008; Ho et al., 2015; Reinertsen et al., 2017; Von Ah et al., 2008; Yee et al., 2017).
Although longitudinal studies evaluated for changes in average fatigue severity during chemotherapy, the wide range in the timing of the assessments, such as every third chemotherapy cycle (Yeh, 2021), every three months (Von Ah et al., 2008), every six months (Bower et al., 2021), and after completion of treatment (Bower et al., 2014; Cohen et al., 2019; Fagundes et al., 2012; Reinertsen et al., 2017), makes it difficult to determine how stress affects fatigue severity during chemotherapy. For example, in a study that evaluated fatigue severity for six days following a cycle of chemotherapy (Higgins et al., 2008), higher levels of pretreatment distress were associated with increased severity of average fatigue. However, only a single measure of global stress was used in this analysis. None of the studies evaluated for differences in three distinct types of stress (i.e., global, cancer-specific, and cumulative life stress) among patients with cancer who had distinct joint morning and evening fatigue profiles. An evaluation of distinct types of stress is important to be able to provide individualized supportive care interventions; interventions may differ for patients with post-traumatic stress disorder (PTSD) and those with adverse childhood experiences (ACEs).
Fatigue and Resilience
Resilience is an individualized, dynamic process of positive adaptation to stressors that can decrease allostatic load (Charney, 2004). In studies that evaluated for associations among resilience, fatigue, and stress in patients during cancer treatment, lower resilience scores were associated with higher levels of fatigue, global stress (Min et al., 2013), and cancer-specific stress (Alarcón et al., 2020). In one systematic review (Tamura et al., 2021), lower levels of resilience were associated with higher levels of depression, anxiety, and fatigue in patients with cancer. However, only 2 of the 39 studies included in this review evaluated for associations between fatigue and resilience (Ristevska-Dimitrovska et al., 2015; Zou et al., 2018). In two additional studies, higher levels of average fatigue were associated with lower levels of resilience (Lin et al., 2020; Öcalan & Üzar-Özçetin, 2022). These findings suggest that decreased resilience is a risk factor for more severe fatigue. Additional research is warranted because the three studies that evaluated patients during chemotherapy were cross-sectional (Öcalan & Üzar-Özçetin, 2021; Ristevska-Dimitrovska et al., 2015; Zou et al., 2018). In addition, the fourth longitudinal study evaluated only patients with colorectal cancer following surgery (Lin et al., 2020).
Fatigue and Coping
Healthy individuals employ coping strategies to respond to stress and maintain homeostasis (Folkman et al., 1986; Lazarus & Folkman, 1984). Coping strategies can be broadly classified as engagement strategies (e.g., positive reframing, seeking instrumental and emotional support) or disengagement strategies (e.g., avoidance, denial) (Carver et al., 1989; Folkman et al., 1986; Langford et al., 2017; Lazarus & Folkman, 1984). Although engagement coping strategies are associated with decreased fatigue severity, across multiple cancer diagnoses (Dsouza et al., 2018; Narayanan et al., 2020; Reuter et al., 2006; van de Wiel et al., 2021; Yeun & Jeon, 2020), the use of disengagement coping strategies is associated with higher levels of average fatigue (Baussard et al., 2022; Bussell & Naus, 2010; Dahal & Meheta, 2018; Dong et al., 2021; Ichikura et al., 2018; Levkovich, 2021). However, because these studies evaluated only average fatigue, no information is available on associations with diurnal variations in fatigue severity.
An examination of differences in stress, resilience, and the use of coping strategies among patients with worse joint morning and evening fatigue profiles may identify modifiable risk factors that can be targeted to decrease both types of physical fatigue (i.e., morning and evening). Therefore, this study aimed to evaluate for differences in global, cancer-specific, and cumulative life stress, as well as resilience and coping, in subgroups of patients (i.e., latent classes) with distinct joint morning and evening fatigue profiles.
Methods
Patients and Settings
Details about the parent longitudinal study, which was guided by the theory of symptom management (Weiss et al., 2023) are published elsewhere (Miaskowski et al., 2014). In brief, eligible patients were aged 18 years or older; had a diagnosis of breast, gastrointestinal, gynecologic, or lung cancer; had received chemotherapy within the preceding four weeks; were scheduled to receive at least two additional cycles of chemotherapy; were able to read, write, and understand English; and gave written informed consent.
Patients were recruited from two National Cancer Institute–designated comprehensive cancer centers, one Veterans Affairs hospital, and four community-based oncology programs. A total of 2,234 patients were approached during their first or second cycle of chemotherapy and 1,343 consented to participate (60% accrual rate). The primary reason indicated for declining to participate in the study was being overwhelmed with their cancer treatment.
Study Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco, and by the institutional review board at each of the study sites. Eligible patients were approached by a research staff member in the infusion unit during their first or second cycle of chemotherapy to discuss the study procedures and their interest in study participation. Written informed consent was obtained from all patients. Patients completed the morning and evening fatigue measures in their homes using paper questionnaires. Measures were completed a total of six times over two cycles of chemotherapy: prior to chemotherapy (assessments 1 and 4), one week following the administration of chemotherapy (assessments 2 and 5), and two weeks following the administration of chemotherapy (assessments 3 and 6). The remaining questionnaires were completed at enrollment (i.e., prior to the second or third cycle of chemotherapy). Medical records were reviewed for disease and treatment information. The 1,334 patients who completed both the morning and evening fatigue measures were included in this analysis.
Measures
Demographic and Clinical Characteristics
Patients completed a demographic questionnaire, the Karnofsky Performance Status Scale (Karnofsky, 1977), and the Self-Administered Comorbidity Questionnaire (Sangha et al., 2003). Medical records were reviewed for disease and treatment information.
Fatigue
The 18-item Lee Fatigue Scale (LFS) was designed to assess physical fatigue and energy (Lee et al., 1991). Each item is rated on a 0–10 numeric rating scale. Total fatigue and energy scores are calculated as the mean of the 13 fatigue items and the mean of the 5 energy items, respectively. Higher scores on the fatigue items indicate greater fatigue severity, and higher scores on the energy items indicate higher levels of energy. Using separate LFS questionnaires, patients were asked to rate each item based on how they felt within 30 minutes of awakening (morning fatigue) and before going to bed (evening fatigue). The LFS has established cutoff scores for clinically meaningful levels of fatigue (3.2 or greater for morning fatigue, 5.6 or greater for evening fatigue) and energy (6.2 or less for morning energy, 3.5 or less for evening energy) (Fletcher et al., 2008). Cronbach’s alphas were 0.96 for morning fatigue and 0.93 for evening fatigue, and they were 0.95 for morning energy and 0.93 for evening energy.
Global Stress
The 14-item Perceived Stress Scale (PSS) was used to measure global perceived stress according to the degree to which life circumstances during the previous week were appraised as stressful (Cohen et al., 1983). Each item is rated on a scale ranging from 0 (never) to 4 (very often). Total scores are summed and can range from 0 to 56. The Cronbach’s alpha for the PSS was 0.85.
Cancer-Specific Stress
The 22-item Impact of Event Scale–Revised (IES-R) was used to measure cancer-related distress (Horowitz et al., 1979). Patients rate each item based on how distressing each potential difficulty was for them during the past week “with respect to their cancer and its treatment.” Three subscales evaluate levels of avoidance, intrusion, and hyperarousal perceived by the patient. For the IES-R total score, sum scores of 24 or greater indicate clinically meaningful post-traumatic symptomatology, and scores of 33 or greater indicate probable PTSD (Creamer et al., 2003). The Cronbach’s alpha for the IES-R total score was 0.92.
Cumulative Life Stress
The 30-item Life Stressor Checklist–Revised (LSC-R) is an index of lifetime trauma exposure (e.g., mugging, the death of a loved one, a sexual assault) (Wolfe & Kimmerling, 1997). The total LSC-R score is obtained by summing the total number of events endorsed. If patients endorse an event, they are asked to indicate how much that stressor affected their life in the past year. These responses are summed to yield a mean “affected sum” score. In addition, a PTSD sum score is created based on the number of positively endorsed items (out of 21) that reflect the Diagnostic and Statistical Manual of Mental Disorders (4th ed.) PTSD Criteria A for having experienced a traumatic event.
Resilience
The 10-item Connor–Davidson Resilience Scale (CDRS) evaluates a patient’s ability to handle adversity (e.g., “I am able to adapt when changes occur”) (Campbell-Sills & Stein, 2007). Total scores range from 0 to 40, with higher scores indicating higher self-perceived resilience. The normative mean score for adults in the United States is 31.8 (SD = 5.4) (Campbell-Sills et al., 2009). The Cronbach’s alpha of the CDRS was 0.9.
Coping Strategies
The 28-item Brief Coping Orientation to Problems Experienced Inventory scale was designed to assess a broad range of coping responses among adults (Carver, 1997; Carver et al., 1989). Each item is rated on a Likert-type scale ranging from 1 (“I haven’t been doing this at all”) to 4 (“I have been doing this a lot”). Higher scores indicate greater use of the various coping strategies. In total, the following 14 dimensions are evaluated using this instrument (with their respective Cronbach’s alphas): self-distraction (alpha = 0.46), active coping (alpha = 0.75), denial (alpha = 0.72), substance use (alpha = 0.87), use of emotional support (alpha = 0.77), use of instrumental support (alpha = 0.77), behavioral disengagement (alpha = 0.57), venting (alpha = 0.65), positive reframing (alpha = 0.79), planning (alpha = 0.74), humor (alpha = 0.83), acceptance (alpha = 0.68), religion (alpha = 0.92), and self-blame (alpha = 0.73). Each dimension is evaluated using two items.
Data Analysis
As previously reported by Wright et al. (2023), latent profile analysis was used to identify subgroups of patients with distinct joint morning and evening fatigue profiles (using the morning and evening LFS scores obtained during the six assessments in a single latent profile analysis). Mplus, version 8.4 (Muthén & Muthén, 1998–2017), was used to conduct the analysis. This approach provides a profile description of these two symptoms with parallel profiles over time.
Additional data were analyzed using IBM SPSS Statistics, version 28.0. Differences among the groups with distinct joint morning and evening fatigue profiles in global, cancer-specific, and cumulative life stress, as well as in resilience and the use of coping strategies at enrollment, were evaluated using parametric (e.g., analysis of variance) and nonparametric (e.g., Kruskal–Wallis) tests. A Bonferroni-corrected p value of p < 0.008 was considered statistically significant for the pairwise contrasts (0.05/6 possible pairwise contrasts).
Results
Sample Characteristics
As previously noted by Wright et al. (2023), in brief, compared to the both low class, patients in the both high class were younger, were less likely to be married or partnered, were more likely to live alone, had a higher comorbidity burden, and had a lower functional status. In addition, patients in the both high class reported significantly higher anxiety, depression, sleep disturbance, and pain scores, and lower quality-of-life scores.
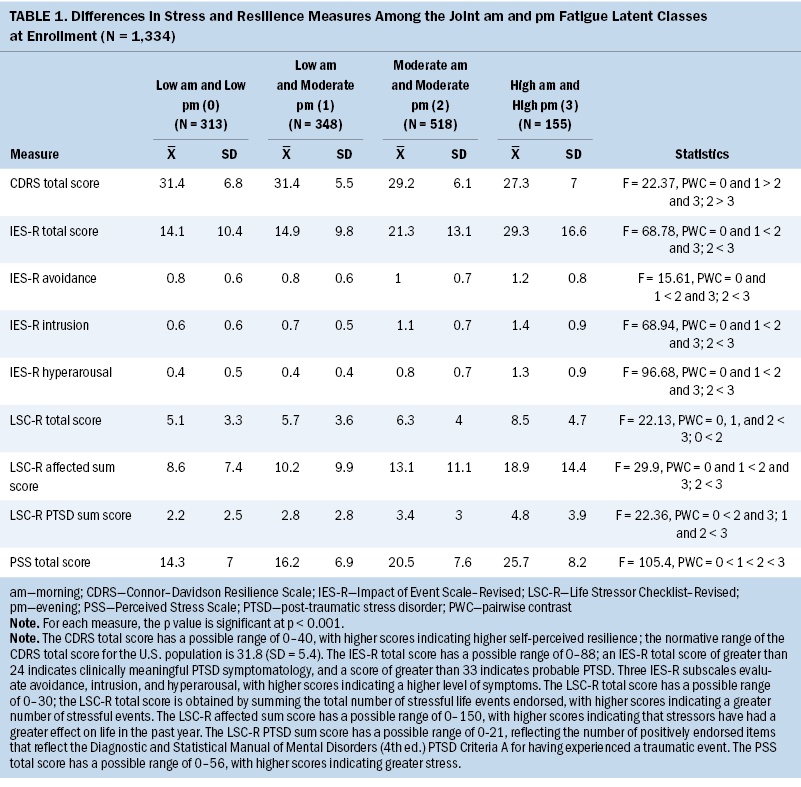
Stress and Resilience
As shown in Table 1, across the four classes, significant increases in PSS scores were found to be consistent with increased morning and/or evening fatigue. PSS scores occurred in the following order, from lowest to highest: both low, low morning and moderate evening, both moderate, and both high. Compared to the both low class and the low morning and moderate evening class, the other two classes had higher IES-R subscale and total scores. Compared to the other three classes, the both high class had higher LSC-R total scores. Compared to the both low class, the both moderate and both high classes had higher LSC-R affected and PTSD sum scores. In terms of resilience, compared to the both low and the low morning and moderate evening classes, the other two classes had lower CDRS scores.
Occurrence of Stressful Life Events
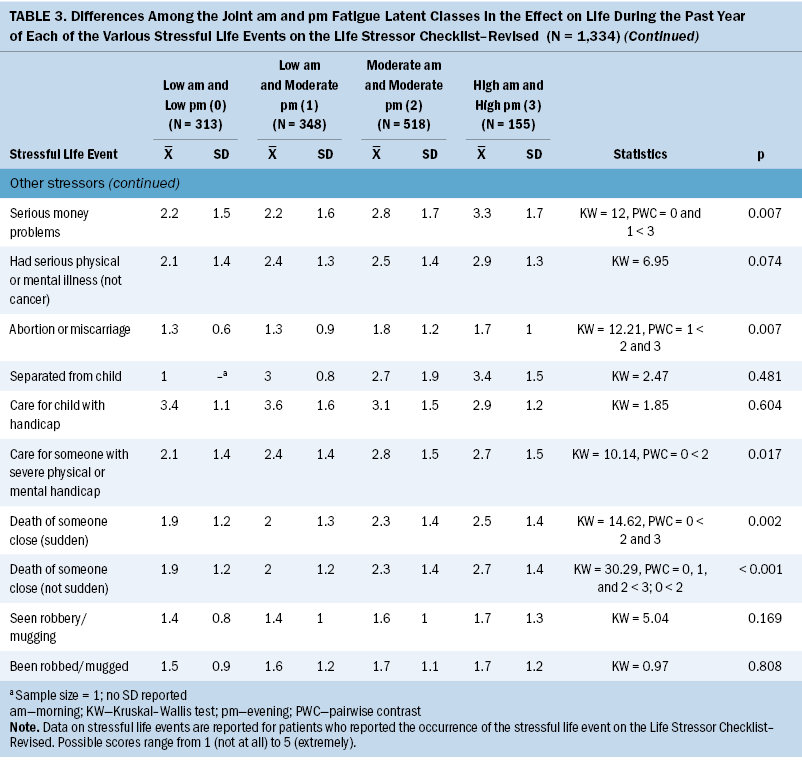
Significant differences were found among the joint morning and evening fatigue classes for all the interpersonal violence, abuse, and neglect stressors evaluated using the LSC-R (see Table 2). Compared to the other three classes, the both high class was more likely to report family violence in childhood, emotional abuse, and physical abuse at younger than age 16 years and at age 16 years or older. Compared to the both low and low morning and moderate evening classes, the both high class was more likely to report being exposed to physical neglect and forced to have sex at age 16 years or older. Compared to the low morning and moderate evening class, the both high class was more likely to report being forced to sexually touch at younger than age 16 years and at age 16 years or older and to have been forced to have sex at younger than age 16 years. Compared to the both low class, the both moderate class was more likely to report emotional abuse and being forced to sexually touch at age 16 years or older.
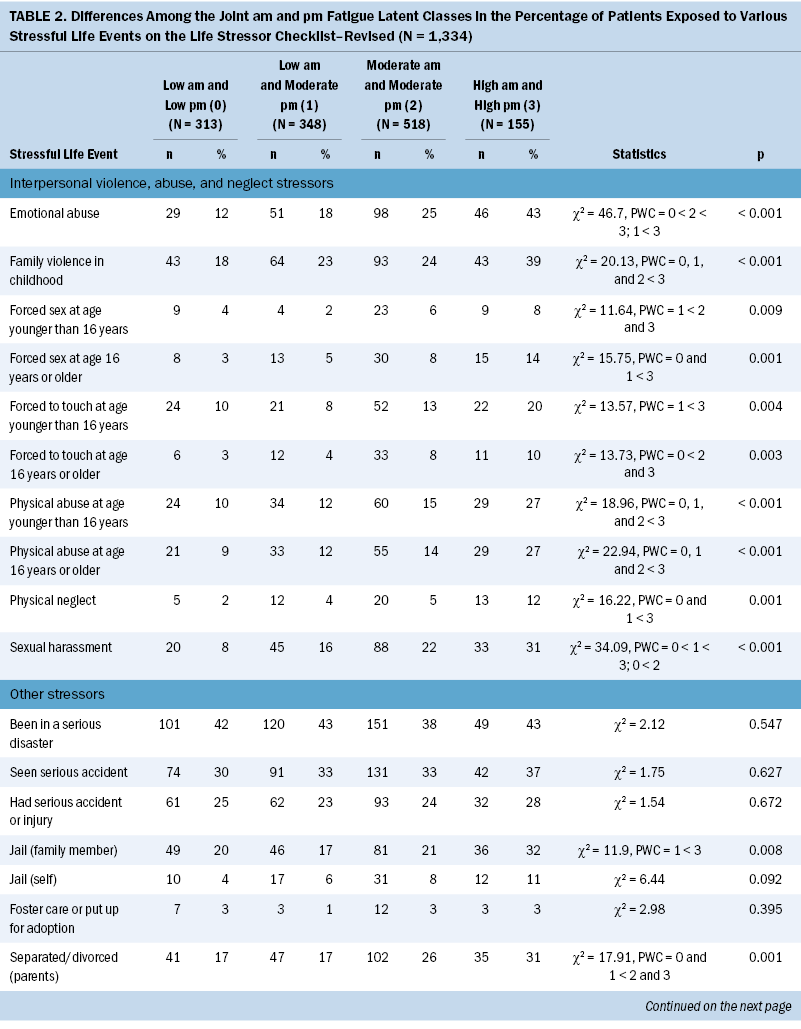
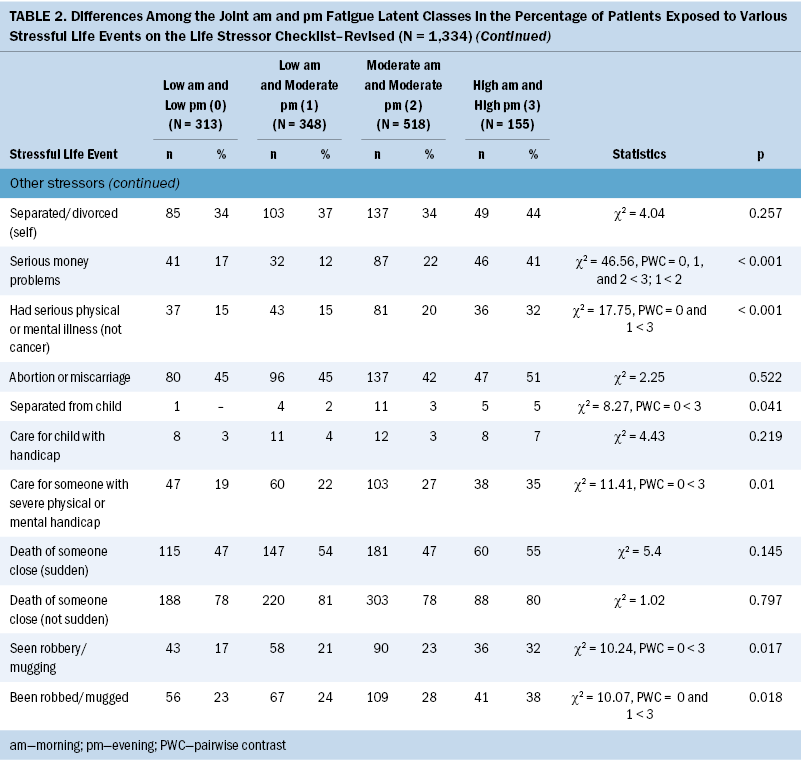
In terms of other stressful life events (SLEs), compared to the both low class, the both high class was more likely to report parental separation or divorce; serious physical or mental illness other than cancer; separation from their child; caring for someone with a severe physical or mental handicap; and having seen a robbery or a mugging and having been robbed or mugged. Compared to the low morning and moderate evening class, the both high class was more likely to report a family member being incarcerated, having a serious physical or mental illness other than cancer, and having been robbed or mugged. Compared to the other three classes, the both high class reported serious money problems. Compared to the low morning and moderate evening class, the both moderate class was more likely to report parental separation or divorce and serious money problems.
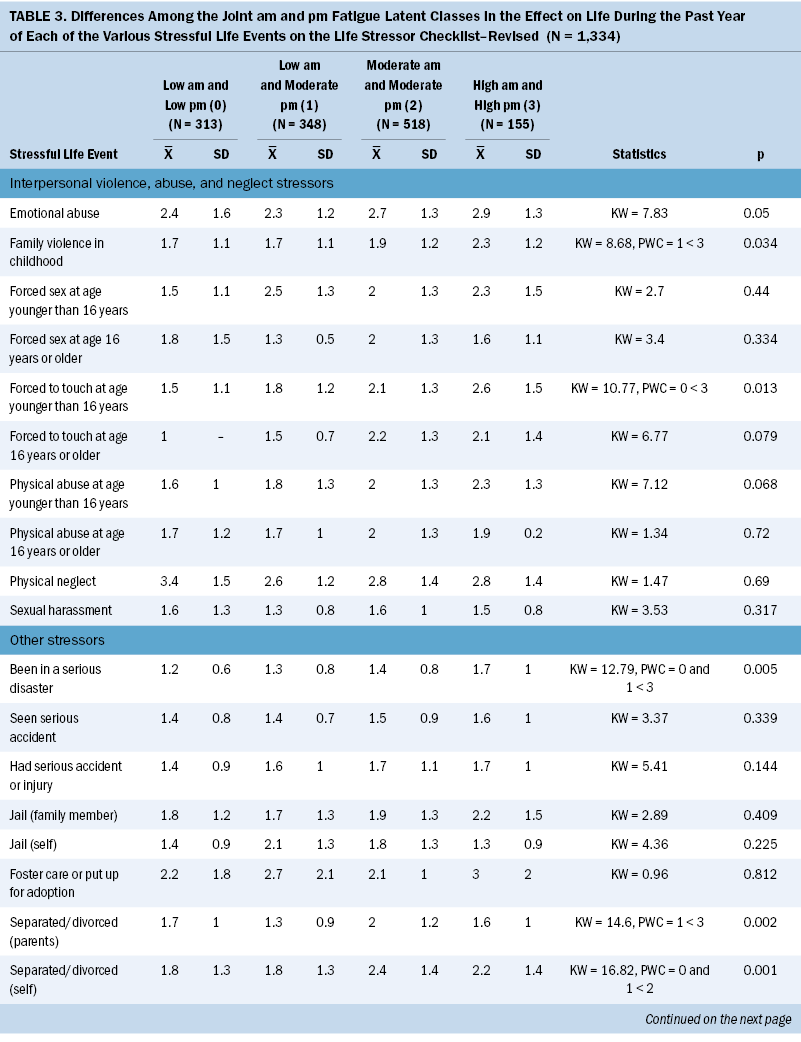
Effect of SLEs
Significant differences were found among the joint morning and evening fatigue classes in the effect of various SLEs (see Table 3). Compared to the both low class, the both high class was more likely to have been affected by being forced to sexually touch at age younger than 16 years, being in a serious disaster, and having serious money problems. Compared to the low morning and moderate evening class, the both high class was more likely to have been affected by exposure to family violence during childhood, a serious disaster, parental separation or divorce, and serious money problems. Compared to the low morning and moderate evening class, the both moderate and both high classes were more likely to have been affected by an abortion or miscarriage. Compared to the both low class, the both moderate class was more likely to have been affected by caring for someone with a physical or mental handicap. Compared to the both low class, the both moderate and both high classes were more likely to have been affected by someone close to them dying suddenly. Compared to the other three classes, the both high class was more likely to have been affected by someone close to them dying during the past year.
Coping Strategies
Regarding engagement coping strategies, compared to the low morning and moderate evening class, the both high class was less likely to use active coping and the both moderate class was less likely to use acceptance (see Table 4). Compared to the both low class, the other three classes were more likely to use planning. Compared to the both low class, the low morning and moderate evening class was more likely to use emotional support and the both moderate class was more likely to use instrumental support.
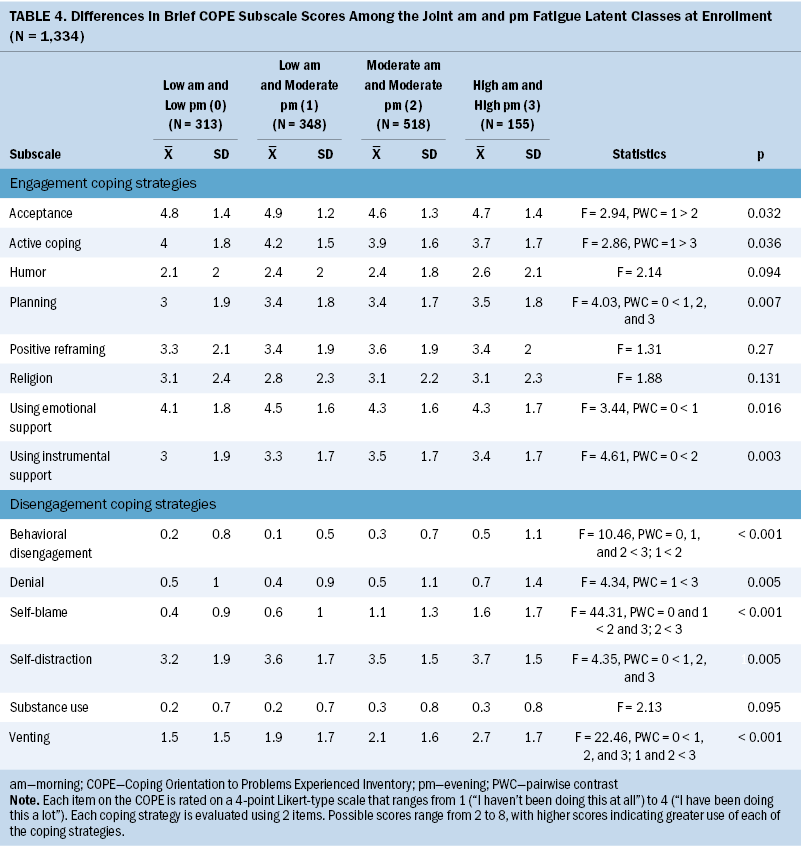
In terms of disengagement coping strategies, compared to the both low class, the other three classes were more likely to use self-distraction and venting. Compared to the low morning and moderate evening class, the both high class was more likely to use venting and denial and the both moderate class was more likely to use behavioral disengagement. Compared to the other three classes, the both high class was more likely to use behavioral disengagement and self-blame. Compared to the both low and the low morning and moderate evening classes, the both moderate class was more likely to use self-blame.
Discussion
Building on the authors’ previous analysis of morning and evening fatigue as individual symptoms (Wright, Cooper, et al., 2017; Wright et al., 2019, 2020) and the authors’ findings on various demographic, clinical, and symptom characteristics associated with joint morning and evening fatigue profiles (Wright et al., 2023), this study provides new insights into the association between joint fatigue profiles and global stress, cancer-specific stress, and the occurrence and effect of SLEs and ACEs, as well as resilience and coping. Patients in the both high class reported the highest stress scores, the highest occurrence rates for and effects from a number of ACEs, the lowest resilience scores, and higher use of disengagement coping strategies. The remainder of the discussion places these findings in the context of the extant literature, describes relevant mechanisms to support the findings, and provides recommendations for research and clinical practice.
Stress
The robust associations between all the stress measures and the worst joint morning and evening fatigue profiles can be partially explained by the fact that cancer and its treatment are significant stressors (Merluzzi et al., 2022) associated with dysregulation of the hypothalamic–pituitary–adrenal axis (Bower, 2019; Ravi et al., 2021). In terms of global stress, the both moderate and both high classes had PSS scores that are comparable to those of pregnant women in neighborhoods with high rates of violent crime (Shannon et al., 2020) and to those of men and women within one month after myocardial infarction (Xu et al., 2015). Higher levels of global stress were associated with greater fatigue severity in adults with inflammatory bowel disease (Luber et al., 2022), unexplained chronic fatigue (Campbell et al., 2017), and rheumatic conditions (Hung et al., 2020). Chronic stress activates the immune system with resultant increases in allostatic load and inflammation (Ravi et al., 2021). These processes may be common mechanisms that underlie these conditions, as well as the fatigue reported by patients and other chronic conditions. Future studies need to evaluate for additive or synergistic interactions between stress and inflammation and their contributions to the occurrence and severity of morning and/or evening fatigue.
In terms of cancer-specific stress, given the significant impact of a cancer diagnosis and its treatments (Merluzzi et al., 2022), the higher subscale and total IES-R scores for the both high class are not unexpected. The IES-R total score was higher than the clinically meaningful cutoff score for PTSD symptomatology of 24 or greater established in studies of earthquake survivors and survivors of the Tokyo subway sarin gas attack (Asukai et al., 2002; Creamer et al., 2003), and it met the criteria for subsyndromal PTSD (Weiss, 2007). The higher scores for the IES-R avoidance, intrusion, and hyperarousal subscales reported by the both high class suggest a PTSD diagnosis (Frances et al., 1995).
Exposure to ACEs during brain development (i.e., experiencing SLEs at age younger than 16 years) may alter neuroimmune interactions that increase the risk for PTSD (Andersen, 2022). The both high class endorsed the highest occurrence rates for several ACEs, including family violence in childhood (n = 43, 39%); emotional abuse (n = 46, 43%); physical neglect (n = 13, 12%); and physical abuse (n = 29, 27%), forced touching (n = 22, 20%), and forced sex (n = 9, 8%) at age younger than 16 years. These findings are consistent with prior research that identified associations between average fatigue and multiple ACEs in healthy college students (Kalmakis et al., 2022), adults with multiple sclerosis (Pust et al., 2021), patients with chronic fatigue syndrome (De Venter et al., 2017), and women with breast cancer (Bower et al., 2014, 2021). The synergy between the occurrence of ACEs (Briggs et al., 2021; Parnes & Schwartz, 2022) and worse joint morning and evening fatigue profiles warrants additional investigation in patients with cancer. One potential explanation for this association is that ACEs increase allostatic load (Bower et al., 2014; Lacey et al., 2020; Steel et al., 2020). In addition, increasing evidence suggests that various neuroimmune interactions contribute to a higher symptom burden in patients with cancer (Bower, 2019; Scheff & Saloman, 2021).
Taken together, these findings suggest that interventions aimed at decreasing stress, such as exercise (Oppegaard et al., 2021), mindfulness meditation (Xie et al., 2020), and yoga (Danhauer et al., 2019; Selvan et al., 2022), may decrease morning and evening fatigue severity. Clinicians who care for patients with cancer need to evaluate their levels of stress and ACE exposures and provide referrals to psychological services. Given the paucity of research on SLEs and ACEs in patients with cancer, studies are warranted to confirm or refute these findings and evaluate the effects of stress reduction or resilience-enhancing interventions to decrease morning and/or evening fatigue severity.
Resilience
Resilience is one factor that influences an individual’s susceptibility to the adverse effects of SLEs (Macía et al., 2020; Weber & O’Brien, 2017). For the both moderate and both high classes, their CDRS scores were below the normative score for the general population of the United States (Campbell-Sills et al., 2009). These findings are consistent with two studies of patients with gastric cancer that found that resilience was negatively correlated with fatigue severity and explained 15% (Zou et al., 2018) to 16% (Tian & Hong, 2014) of the variance in average fatigue. Higher levels of resilience are associated with more adaptive coping to physical and emotional challenges (Macía et al., 2020). Interventions to increase resilience, such as making meaning of the cancer experience (Seiler & Jenewein, 2019), social support (Abbott et al., 2021; Borgi et al., 2020; Seiler & Jenewein, 2019), and attention and interpretation therapy (Lin et al., 2020), may decrease stress, alter neuroimmune responses (Borgi et al., 2020), and decrease fatigue. To the authors’ knowledge, this study is the first to evaluate the impact of resilience on the co-occurrence of morning and evening fatigue.
Coping Strategies
Compared to the both low class, the other three classes were more likely to use planning as a coping strategy. Given that the use of planning was found to help patients with cancer adjust to their current situation (Nilsen et al., 2021), these findings may reflect patients’ efforts to mitigate the effects of morning and/or evening fatigue.
Compared to the other three classes, the both high class used more disengagement coping strategies. Of note, in studies of patients with end-stage kidney disease (Picariello et al., 2018), patients during cancer chemotherapy (Dahal & Meheta, 2018), and healthy working adults (Otsuka et al., 2009), disengagement coping strategies were used to decrease fatigue. Although engagement coping strategies are considered adaptive and encourage post-traumatic growth (Nik Jaafar et al., 2021; Shand et al., 2015), disengagement coping strategies were found to mediate the impact of ACEs on cancer-specific stress (Langford et al., 2017).
It is important to note that patients employ multiple coping strategies (Leonidou et al., 2019). Several qualitative studies highlight the need for clinicians to be aware of patients’ beliefs about their coping efficacy (Dong et al., 2021; Yeun & Jeon, 2020). Higher levels of coping efficacy are associated with higher levels of emotional and physical well-being (Merluzzi et al., 2022). Therefore, additional research is warranted on the efficacy of interventions that support patients’ coping efficacy and the development of more robust engagement strategies, such as personalizing support (Schellekens et al., 2021) and solution-focused interventions (Wang et al., 2021), to reduce fatigue and various types of stress.
Strengths and Limitations
The strengths of this study include a large sample size, patients with heterogeneous types of cancer, and the evaluation of three different types of stress, as well as resilience and coping. However, stress, resilience, and coping strategies were evaluated only upon enrollment. Therefore, associations between changes in the co-occurrence of morning and evening fatigue and changes in the various stress measures warrant evaluation during and following the completion of chemotherapy. In addition, an evaluation of stress biomarkers will increase knowledge of the mechanisms that underlie the relationships between the co-occurrence of morning and evening fatigue and stress. Equally important, future studies need to evaluate the impact of various social determinants of health (e.g., social support, neighborhood environments) that may contribute to both fatigue and stress.
Conclusion and Implications for Practice
This study is the first to evaluate for associations between higher levels of joint morning and evening fatigue profiles in the same patients as well as levels of different types of stress, resilience, and use of coping strategies. In addition to the clinical implications noted previously, a coordinated approach is needed to decrease stress and provide supportive services to patients undergoing chemotherapy. Clinical assessment of patients’ level of resilience, preferred coping strategies, and perceived coping efficacy has the potential to inform personalized supportive interventions such as referrals for psychosocial support, stress management, resilience training, and exercise. The diurnal nature of fatigue suggests that the timing of personalized interventions to decrease fatigue needs to be considered. Additional studies are needed to evaluate the prescription of personalized strategies based on a patient’s morning and evening fatigue profile to decrease both fatigue and stress.
About the Authors
Fay Wright, RN, PhD, APRN-BC, was, at the time of this writing, an assistant professor in the Rory Meyers College of Nursing at New York University in New York City, and is currently a nurse scientist at Northern Westchester Hospital, Northwell Health; Bruce A. Cooper, PhD, is a data analyst III and senior statistician in the Department of Physiological Nursing in the School of Nursing at the University of California, San Francisco; Marilyn J. Hammer, RN, PhD, DC, FAAN, is the director of the Phyllis F. Cantor Center for Research in Nursing and Patient Care Services at the Dana-Farber Cancer Institute in Boston, MA; Steven M. Paul, PhD, is a research data analyst III in the Department of Physiological Nursing in the School of Nursing at the University of California, San Francisco; Yvette P. Conley, PhD, FAAN, is a professor in the Department of Health Promotion and Development in the School of Nursing at the University of Pittsburgh in Pennsylvania; and Jon D. Levine, MD, PhD, is a professor in the Department of Oral and Maxillofacial Surgery in the School of Dentistry, Christine Miaskowski, RN, PhD, FAAN, is a professor in the Department of Physiological Nursing in the School of Nursing, and Kord M. Kober, PhD, is an associate professor in the Department of Physiological Nursing in the School of Nursing, all at the University of California, San Francisco. This research was funded, in part, by a grant from the National Cancer Institute (CA134900). Wright received research funding from New York University Langone Health’s Clinical and Translational Science Institute KL2 Scholar Award (TR 1446-6). Miaskowski is an American Cancer Society Clinical Research Professor. Wright, Conley, Miaskowski, and Kober contributed to the conceptualization and design. Wright, Hammer, and Miaskowski completed the data collection. Wright, Paul, and Miaskowski provided statistical support. Wright, Cooper, and Miaskowski provided the analysis. Wright, Hammer, Conley, Levine, and Miaskowski contributed to the manuscript preparation. Wright can be reached at fwright4@northwell.edu, with copy to ONFEditor@ons.org. (Submitted August 2023. Accepted November 29, 2023.)
References
Abbott, L.S., Graven, L.J., Schluck, G., & Williams, K.J. (2021). Stress, social support, and resilience in younger rural women: A structural equation model. Healthcare, 9(7), 812. https://doi.org/10.3390/healthcare9070812
Alarcón, R., Cerezo, M.V., Hevilla, S., & Blanca, M.J. (2020). Psychometric properties of the Connor-Davidson Resilience Scale in women with breast cancer. International Journal of Clinical and Health Psychology, 20(1), 81–89. https://doi.org/10.1016/j.ijchp.2019.11.001
Andersen, S.L. (2022). Neuroinflammation, early-life adversity, and brain development. Harvard Review of Psychiatry, 30(1), 24–39. https://doi.org/10.1097/hrp.0000000000000325
Asukai, N., Kato, H., Kawamura, N., Kim, Y., Yamamoto, K., Kishimoto, J., . . . Nishizono-Maher, A. (2002). Reliability and validity of the Japanese-language version of the Impact of Event Scale–Revised (IES-R-J): Four studies of different traumatic events. Journal of Nervous and Mental Disease, 190(3), 175–182. https://doi.org/10.1097/00005053-200203000-00006
Baussard, L., Proust-Lima, C., Philipps, V., Portales, F., Ychou, M., Mazard, T., & Cousson-Gélie, F. (2022). Determinants of distinct trajectories of fatigue in patients undergoing chemotherapy for a metastatic colorectal cancer: 6-month follow-up using growth mixture modeling. Journal of Pain and Symptom Management, 63(1), 140–150. https://doi.org/10.1016/j.jpainsymman.2021.06.019
Bean, H.R., Diggens, J., Ftanou, M., Weihs, K.L., Stanton, A.L., & Wiley, J.F. (2021). Insomnia and fatigue symptom trajectories in breast cancer: A longitudinal cohort study. Behavioral Sleep Medicine, 19(6), 814–827. https://doi.org/10.1080/15402002.2020.1869005
Borgi, M., Collacchi, B., Ortona, E., & Cirulli, F. (2020). Stress and coping in women with breast cancer: Unravelling the mechanisms to improve resilience. Neuroscience and Biobehavioral Reviews, 119, 406–421. https://doi.org/10.1016/j.neubiorev.2020.10.011
Bower, J.E. (2019). The role of neuro-immune interactions in cancer-related fatigue: Biobehavioral risk factors and mechanisms. Cancer, 125(3), 353–364. https://doi.org/10.1002/cncr.31790
Bower, J.E., Asher, A., Garet, D., Petersen, L., Ganz, P.A., Irwin, M.R., . . . Crespi, C.M. (2019). Testing a biobehavioral model of fatigue before adjuvant therapy in women with breast cancer. Cancer, 125(4), 633–641. https://doi.org/10.1002/cncr.31827
Bower, J.E., Crosswell, A.D., & Slavich, G.M. (2014). Childhood adversity and cumulative life stress: Risk factors for cancer-related fatigue. Clinical Psycholological Science, 2(1), 108–115. https://doi.org/10.1177/2167702613496243
Bower, J.E., Ganz, P.A., Irwin, M.R., Cole, S.W., Garet, D., Petersen, L., . . . Crespi, C.M. (2021). Do all patients with cancer experience fatigue? A longitudinal study of fatigue trajectories in women with breast cancer. Cancer, 127(8), 1334–1344. https://doi.org/10.1002/cncr.33327
Briggs, E.C., Amaya-Jackson, L., Putnam, K.T., & Putnam, F.W. (2021). All adverse childhood experiences are not equal: The contribution of synergy to adverse childhood experience scores. American Psychologist, 76(2), 243–252. https://doi.org/10.1037/amp0000768
Bussell, V.A., & Naus, M.J. (2010). A longitudinal investigation of coping and posttraumatic growth in breast cancer survivors. Journal of Psychosocial Oncology, 28(1), 61–78. https://doi.org/10.1080/07347330903438958
Campbell, R., Tobback, E., Delesie, L., Vogelaers, D., Mariman, A., & Vansteenkiste, M. (2017). Basic psychological need experiences, fatigue, and sleep in individuals with unexplained chronic fatigue. Stress and Health, 33(5), 645–655. https://doi.org/10.1002/smi.2751
Campbell-Sills, L., Forde, D.R., & Stein, M.B. (2009). Demographic and childhood environmental predictors of resilience in a community sample. Journal of Psychiatric Research, 43(12), 1007–1012. https://doi.org/10.1016/j.jpsychires.2009.01.013
Campbell-Sills, L., & Stein, M.B. (2007). Psychometric analysis and refinement of the Connor-Davidson Resilience Scale (CD-RISC): Validation of a 10-item measure of resilience. Journal of Traumatic Stress, 20(6), 1019–1028. https://doi.org/10.1002/jts.20271
Carver, C.S. (1997). You want to measure coping but your protocol’s too long: Consider the brief COPE. International Journal of Behavioral Medicine, 4(1), 92–100. https://doi.org/10.1207/s15327558ijbm0401_6
Carver, C.S., Scheier, M.F., & Weintraub, J.K. (1989). Assessing coping strategies: A theoretically based approach. Journal of Personality and Social Psychology, 56(2), 267–283. https://doi.org/10.1037//0022-3514.56.2.267
Charney, D.S. (2004). Psychobiological mechanisms of resilience and vulnerability: Implications for successful adaptation to extreme stress. American Journal of Psychiatry, 161(2), 195–216. https://doi.org/10.1176/appi.ajp.161.2.195
Cohen, M., Levkovich, I., Pollack, S., & Fried, G. (2019). Stability and change of postchemotherapy symptoms in relation to optimism and subjective stress: A prospective study of breast cancer survivors. Psycho-Oncology, 28(10), 2017–2024. https://doi.org/10.1002/pon.5185
Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396.
Creamer, M., Bell, R., & Failla, S. (2003). Psychometric properties of the Impact of Event Scale–Revised. Behaviour Research and Therapy, 41(12), 1489–1496. https://doi.org/10.1016/j.brat.2003.07.010
Dahal, A., & Meheta, R.K. (2018). Fatigue experience and coping strategies among cancer patients receiving chemotherapy. Journal of Nepal Health Research Council, 16(3), 285–290. https://ncbi.nlm.nih.gov/pubmed/30455487
Danhauer, S.C., Addington, E.L., Cohen, L., Sohl, S.J., Van Puymbroeck, M., Albinati, N.K., & Culos-Reed, S.N. (2019). Yoga for symptom management in oncology: A review of the evidence base and future directions for research. Cancer, 125(12), 1979–1989. https://doi.org/10.1002/cncr.31979
De Venter, M., Illegems, J., Van Royen, R., Moorkens, G., Sabbe, B.G.C., & Van Den Eede, F. (2017). Differential effects of childhood trauma subtypes on fatigue and physical functioning in chronic fatigue syndrome. Comprehensive Psychiatry, 78, 76–82.
Dhruva, A., Aouizerat, B.E., Cooper, B., Paul, S.M., Dodd, M., West, C., . . . Miaskowski, C. (2013). Differences in morning and evening fatigue in oncology patients and their family caregivers. European Journal of Oncology Nursing, 17(6), 841–848. https://doi.org/10.1016/j.ejon.2013.06.002
Dimsdale, J.E., Ancoli-Israel, S., Ayalon, L., Elsmore, T.F., & Gruen, W. (2007). Taking fatigue seriously, II: Variability in fatigue levels in cancer patients. Psychosomatics, 48(3), 247–252. https://doi.org/10.1176/appi.psy.48.3.247
Dong, X., Peng, J., Li, X., Zhao, Q., & Zhang, X. (2021). Home coping strategies for fatigue used by patients with lung cancer receiving chemotherapy in rural China: A qualitative study. Journal of Nursing Research, 29(6), e178. https://doi.org/10.1097/jnr.0000000000000453
Dsouza, A., Kamboj, R., Mandavkar, S., Chavan, N., Ramaswamy, A., & Ostwal, V. (2018). An evaluation of early-onset fatigue and the related coping strategies in patients with gastrointestinal cancer: A prospective pilot study. Indian Journal of Cancer, 55(2), 162–165. https://doi.org/10.4103/ijc.IJC_568_17
Fagundes, C.P., Lindgren, M.E., Shapiro, C.L., & Kiecolt-Glaser, J.K. (2012). Child maltreatment and breast cancer survivors: Social support makes a difference for quality of life, fatigue and cancer stress. European Journal of Cancer, 48(5), 728–736.
Fletcher, B.S., Paul, S.M., Dodd, M.J., Schumacher, K., West, C., Cooper, B., . . . Miaskowski, C.A. (2008). Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. Journal of Clinical Oncology, 26(4), 599–605. https://doi.org/10.1200/JCO.2007.12.2838
Folkman, S., Lazarus, R.S., Gruen, R.J., & DeLongis, A. (1986). Appraisal, coping, health status, and psychological symptoms. Journal of Personality and Social Psychology, 50(3), 571–579.
Frances, A., First, M.B., & Pincus, H.A. (1995). DSM-IV guidebook. American Psychiatric Association.
Galatzer-Levy, I.R., Huang, S.H., & Bonanno, G.A. (2018). Trajectories of resilience and dysfunction following potential trauma: A review and statistical evaluation. Clinical Psychology Review, 63, 41–55. https://doi.org/10.1016/j.cpr.2018.05.008
Higgins, S.C., Montgomery, G.H., Raptis, G., & Bovbjerg, D.H. (2008). Effect of pretreatment distress on daily fatigue after chemotherapy for breast cancer. Journal of Oncology Practice, 4(2), 59–63. https://doi.org/10.1200/JOP.0822002
Ho, R.T.H, Kwan, T.T.C., Cheung, I.K.M., Chan, C.K.P., Lo, P.H.Y., Yip, P.S.F., . . . Chan, C.L.W. (2015). Association of fatigue with perceived stress in Chinese women with early stage breast cancer awaiting adjuvant radiotherapy. Stress and Health, 31(3), 214–221. https://doi.org/10.1002/smi.2548
Horowitz, M., Wilner, N., & Alvarez, W. (1979). Impact of Event Scale: A measure of subjective stress. Psychosomatic Medicine, 41(3), 209–218. https://doi.org/10.1097/00006842-197905000-00004
Hung, H.-M., Chen, M.-F., & Chen, C.-H. (2020). Impacts of fatigue, stress, and perceived health status on women with rheumatic diseases: A comparison study. Journal of Nursing Research, 28(3), e89. https://doi.org/10.1097/jnr.0000000000000354
Ichikura, K., Yamashita, A., Sugimoto, T., Kishimoto, S., & Matsushima, E. (2018). Patterns of stress coping and depression among patients with head and neck cancer: A Japanese cross-sectional study. Psycho-Oncology, 27(2), 556–562. https://doi.org/10.1002/pon.4549
Kalmakis, K.A., Kent, N.M., Alhowaymel, F., & Chiodo, L.M. (2022). Perceived stress, fatigue symptoms, and posttraumatic stress disorder symptoms among young adult college students. Journal of Child and Adolescent Psychiatric Nursing, 35(1), 60–67. https://doi.org/10.1111/jcap.12352
Karnofsky, D. (1977). Performance scale. Plenum Press.
Kober, K.M., Harris, C., Conley, Y.P., Dhruva, A., Dokiparthi, V., Hammer, M.J., . . . Miaskowski, C. (2023). Perturbations in common and distinct inflammatory pathways associated with morning and evening fatigue in outpatients receiving chemotherapy. Cancer Medicine, 12(6), 7369–7380. https://doi.org/10.1002%2Fcam4.5435
Kop, W.J., & Kupper, H.M. (2016). Fatigue and stress. In G. Fink (Ed.), Stress: Concepts, cognition, emotion, and behavior (pp. 345–350). Academic Press. https://doi.org/10.1016/b978-0-12-800951-2.00043-1
Lacey, R.E., Pinto Pereira, S.M., Li, L., & Danese, A. (2020). Adverse childhood experiences and adult inflammation: Single adversity, cumulative risk and latent class approaches. Brain, Behavior, and Immunity, 87, 820–830. https://doi.org/10.1016/j.bbi.2020.03.017
Langford, D.J., Cooper, B., Paul, S., Humphreys, J., Keagy, C., Conley, Y.P., . . . Dunn, L.B. (2017). Evaluation of coping as a mediator of the relationship between stressful life events and cancer-related distress. Health Psychology, 36(12), 1147–1160. https://doi.org/10.1037/hea0000524
Lazarus, R.S., & Folkman, S. (1984). Stress, appraisal, and coping. Springer.
Lee, K.A., Hicks, G., & Nino-Murcia, G. (1991). Validity and reliability of a scale to assess fatigue. Psychiatry Research, 36(3), 291–298. https://doi.org/10.1016/0165-1781(91)90027-m
Leonidou, C., Panayiotou, G., Bati, A., & Karekla, M. (2019). Coping with psychosomatic symptoms: The buffering role of psychological flexibility and impact on quality of life. Journal of Health Psychology, 24(2), 175–187. https://doi.org/10.1177/1359105316666657
Levkovich, I. (2021). Coping strategies and their impact on emotional distress and fatigue among breast cancer survivors: A cross-sectional survey. Cancer Journal, 27(2), 83–89. https://doi.org/10.1097/PPO.0000000000000505
Lin, C., Diao, Y., Dong, Z., Song, J., & Bao, C. (2020). The effect of attention and interpretation therapy on psychological resilience, cancer-related fatigue, and negative emotions of patients after colon cancer surgery. Annals of Palliative Medicine, 9(5), 3261–3270. https://doi.org/10.21037/apm-20-1370
Luber, R.P., Duff, A., Pavlidis, P., Honap, S., Meade, S., Ray, S., . . . Irving, P.M. (2022). Depression, anxiety, and stress among inflammatory bowel disease patients during COVID-19: A UK cohort study. JGH Open, 6(1), 76–84. https://doi.org/10.1002/jgh3.12699
Macía, P., Barranco, M., Gorbeña, S., & Iraurgi, I. (2020). Expression of resilience, coping and quality of life in people with cancer. PLOS ONE, 15(7), e0236572. https://doi.org/10.1371/journal.pone.0236572
Merluzzi, T.V., Zhang, G., Philip, E.J., Lee, D., & Salamanca-Balen, N. (2022). Discerning critical stressors and resources in the lives of cancer patients: A multivariate analysis of targets of intervention for enhancing cancer care and quality of life. Psycho-Oncology, 31(7), 1186–1195. https://doi.org/10.1002/pon.5906
Miaskowski, C., Cooper, B.A., Melisko, M., Chen, L.-M., Mastick, J., West, C., . . . Aouizerat, B.E. (2014). Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer, 120(15), 2371–2378. https://doi.org/10.1002/cncr.28699
Min, J.-A., Yoon, S., Lee, C.-U., Chae, J.-H., Lee, C., Song, K.-Y., & Kim, T.-S. (2013). Psychological resilience contributes to low emotional distress in cancer patients. Supportive Care in Cancer, 21(9), 2469–2476. https://doi.org/10.1007/s00520-013-1807-6
Muthén, L.K., & Muthén, B.O. (1998-2017). Mplus user’s guide (8th ed.). Muthén and Muthén.
Narayanan, S., Milbury, K., Wagner, R., & Cohen, L. (2020). Religious coping in cancer: A quantitative analysis of expressive writing samples from patients with renal cell carcinoma. Journal of Pain and Symptom Management, 60(4), 737–745.E3. https://doi.org/10.1016/j.jpainsymman.2020.04.029
National Comprehensive Cancer Network. (2023). NCCN Clinical Practice Guideline in Oncology (NCCN Guidelines®): Cancer-related fatigue [v.2.2024]. https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf
Nik Jaafar, N.R., Abd Hamid, N., Hamdan, N.A., Rajandram, R.K., Mahadevan, R., Mohamad Yunus, M.R., . . . Leong Bin Abdullah, M.F.I. (2021). Posttraumatic growth and coping strategies among patients with head and neck cancer: Do approach coping and avoidant coping predict posttraumatic growth over time? Frontiers in Psychology, 12, 716674. https://doi.org/10.3389/fpsyg.2021.716674
Nilsen, M., Stalsberg, R., Sand, K., Haugan, G., & Reidunsdatter, R.J. (2021). Meaning making for psychological adjustment and quality of life in older long-term breast cancer survivors. Frontiers in Psychology, 12, 734198. https://doi.org/10.3389/fpsyg.2021.734198
Öcalan, S., & Üzar-Özçetin, Y.S. (2022). The relationship between rumination, fatigue and psychological resilience among cancer survivors. Journal of Clinical Nursing, 31(23–24), 3595–3604. https://doi.org/10.1111/jocn.16187
Oppegaard, K., Harris, C.S., Shin, J., Paul, S.M., Cooper, B.A., Levine, J.D., . . . Miaskowski, C. (2021). Anxiety profiles are associated with stress, resilience and symptom severity in outpatients receiving chemotherapy. Supportive Care in Cancer, 29(12), 7825–7836. https://doi.org/10.1007/s00520-021-06372-w
Otsuka, Y., Sasaki, T., Iwasaki, K., & Mori, I. (2009). Working hours, coping skills, and psychological health in Japanese daytime workers. Industrial Health, 47(1), 22–32. https://doi.org/10.2486/indhealth.47.22
Parnes, M.F., & Schwartz, S.E.O. (2022). Adverse childhood experiences: Examining latent classes and associations with physical, psychological, and risk-related outcomes in adulthood. Child Abuse and Neglect, 127, 105562. https://doi.org/10.1016/j.chiabu.2022.105562
Picariello, F., Moss-Morris, R., Macdougall, I.C., & Chilcot, J. (2018). ‘It’s when you’re not doing too much you feel tired’: A qualitative exploration of fatigue in end-stage kidney disease. British Journal of Health Psychology, 23(2), 311–333. https://doi.org/10.1111/bjhp.12289
Pietrowsky, R., & Lahl, O. (2008). Diurnal variation of physical and mental fatigue. Sleep and Biological Rhythms, 6(4), 228–233. https://doi.org/10.1111/j.1479-8425.2008.00369.x
Pust, G.E.A., Randerath, J., Goetzmann, L., Weierstall, R., Korzinski, M., Gold, S.M., . . . Schmidt, R. (2021). Association of fatigue severity with maladaptive coping in multiple sclerosis: A data-driven psychodynamic perspective. Frontiers in Neurology, 12, 652177. https://doi.org/10.3389/fneur.2021.652177
Ravi, M., Miller, A.H., & Michopoulos, V. (2021). The immunology of stress and the impact of inflammation on the brain and behavior. BJPsych Advances, 27(Suppl. 3), 158–165. https://doi.org/10.1192/bja.2020.82
Reinertsen, K.V., Engebraaten, O., Loge, J.H., Cvancarova, M., Naume, B., Wist, E., . . . Kiserud, C.E. (2017). Fatigue during and after breast cancer therapy—A prospective study. Journal of Pain and Symptom Management, 53(3), 551–560.
Reuter, K., Classen, C.C., Roscoe, J.A., Morrow, G.R., Kirshner, J.J., Rosenbluth, R., . . . Spiegel, D. (2006). Association of coping style, pain, age and depression with fatigue in women with primary breast cancer. Psycho-Oncology, 15(9), 772–779. https://doi.org/10.1002/pon.1012
Ristevska-Dimitrovska, G., Filov, I., Rajchanovska, D., Stefanovski, P., & Dejanova, B. (2015). Resilience and quality of life in breast cancer patients. Open Access Macedonian Journal of Medical Sciences, 3(4), 727–731. https://doi.org/10.3889/oamjms.2015.128
Sakamoto, N., Takiguchi, S., Komatsu, H., Okuyama, T., Nakaguchi, T., Kubota, Y., . . . Akechi, T. (2017). Supportive care needs and psychological distress and/or quality of life in ambulatory advanced colorectal cancer patients receiving chemotherapy: A cross-sectional study. Japanese Journal of Clinical Oncology, 47(12), 1157–1161. https://doi.org/10.1093/jjco/hyx152
Sangha, O., Stucki, G., Liang, M.H., Fossel, A.H., & Katz, J.N. (2003). The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis and Rheumatism, 49(2), 156–163. https://doi.org/10.1002/art.10993
Scheff, N.N., & Saloman, J.L. (2021). Neuroimmunology of cancer and associated symptomology. Immunology and Cell Biology, 99(9), 949–961. https://doi.org/10.1111/imcb.12496
Schellekens, M.P.J., Bootsma, T.I., van Woezik, R.A.M., & van der Lee, M.L. (2021). Personalizing psychological care for chronic cancer-related fatigue: A case study on symptom dynamics. Journal for Person-Oriented Research, 7(1), 1–13. https://doi.org/10.17505/jpor.2021.23447
Seiler, A., & Jenewein, J. (2019). Resilience in cancer patients. Frontiers in Psychiatry, 10, 208. https://doi.org/10.3389/fpsyt.2019.00208
Selvan, P., Hriso, C., Mitchell, J., & Newberg, A. (2022). Systematic review of yoga for symptom management during conventional treatment of breast cancer patients. Complementary Therapies in Clinical Practice, 48, 101581. https://doi.org/10.1016/j.ctcp.2022.101581
Shand, L.K., Cowlishaw, S., Brooker, J.E., Burney, S., & Ricciardelli, L.A. (2015). Correlates of post-traumatic stress symptoms and growth in cancer patients: A systematic review and meta-analysis. Psycho-Oncology, 24(6), 624–634.
Shannon, M.M., Clougherty, J.E., McCarthy, C., Elovitz, M.A., Nguemeni Tiako, M.J., Melly, S.J., & Burris, H.H. (2020). Neighborhood violent crime and perceived stress in pregnancy. International Journal of Environmental Research and Public Health, 17(15), 5585. https://doi.org/10.3390/ijerph17155585
Shields, G.S., & Slavich, G.M. (2017). Lifetime stress exposure and health: A review of contemporary assessment methods and biological mechanisms. Social and Personality Psychology Compass, 11(8), e12335. https://doi.org/10.1111/spc3.12335
Starr, L.R., Dienes, K., Li, Y.I., & Shaw, Z.A. (2019). Chronic stress exposure, diurnal cortisol slope, and implications for mood and fatigue: Moderation by multilocus HPA-axis genetic variation. Psychoneuroendocrinology, 100, 156–163. https://doi.org/10.1016/j.psyneuen.2018.10.003
Steel, J.L., Antoni, M., Pathak, R., Butterfield, L.H., Vodovotz, Y., Savkova, A., . . . Geller, D.A. (2020). Adverse childhood experiences (ACEs), cell-mediated immunity, and survival in the context of cancer. Brain, Behavior, and Immunity, 88, 566–572. https://doi.org/10.1016/j.bbi.2020.04.050
Tamura, S., Suzuki, K., Ito, Y., & Fukawa, A. (2021). Factors related to the resilience and mental health of adult cancer patients: A systematic review. Supportive Care in Cancer, 29(7), 3471–3486. https://doi.org/10.1007/s00520-020-05943-7
Thorsteinsson, E.B., Brown, R.F., & Owens, M.T. (2019). Modeling the effects of stress, anxiety, and depression on rumination, sleep, and fatigue in a nonclinical sample. Journal of Nervous and Mental Disease, 207(5), 355–359. https://doi.org/10.1097/nmd.0000000000000973
Tian, J., & Hong, J.-S. (2014). Assessment of the relationship between resilience and quality of life in patients with digestive cancer. World Journal of Gastroenterology, 20(48), 18439–18444. https://doi.org/10.3748/wjg.v20.i48.18439
van de Wiel, M., Derijcke, S., Galdermans, D., Daenen, M., Surmont, V., De Droogh, E., . . . Janssens, A. (2021). Coping strategy influences quality of life in patients with advanced lung cancer by mediating mood. Clinical Lung Cancer, 22(2), e146–e152. https://doi.org/10.1016/j.cllc.2020.09.010
Von Ah, D.M., Kang, D.-H., & Carpenter, J.S. (2008). Predictors of cancer-related fatigue in women with breast cancer before, during, and after adjuvant therapy. Cancer Nursing, 31(2), 134–144. https://doi.org/10.1097/01.NCC.0000305704.84164.54
Wang, J., Yin, Y., Li, Y., Yue, X., Qi, X., & Sun, M. (2021). The effects of solution-focused nursing on leukemia chemotherapy patients’ moods, cancer-related fatigue, coping styles, self-efficacy, and quality of life. American Journal of Translational Research, 13(6), 6611–6619. https://pubmed.ncbi.nlm.nih.gov/34306404
Weber, D., & O’Brien, K. (2017). Cancer and cancer-related fatigue and the interrelationships with depression, stress, and inflammation. Journal of Evidence-Based Complementary and Alternative Medicine, 22(3), 502–512. https://doi.org/10.1177/2156587216676122
Weiss, D.S. (2007). The Impact of Event Scale: Revised. In J.P. Wilson & C.S.-K. Tang (Eds.), Cross-cultural assessment of psychological trauma and PTSD (pp. 219–238). Springer. https://psycnet.apa.org/doi/10.1007/978-0-387-70990-1_10
Weiss, S.J., Franck, L.S., Leutwyler, H., Dawson-Rose, C.S., Wallhagen, M.I., Staveski, S.L., . . . Miaskowski, C.A. (2023). Theory of symptom management. In M.J. Smith, P.R. Liehr, & R. Carpenter (Eds.), Middle range theory for nursing (5th ed., pp. 125–141). Springer.
Wolfe, J., & Kimmerling, R. (1997). Gender issues in the assessment of posttraumatic stress disorder. In J.P. Wilson & T.M. Keane (Eds.), Assessing psychological trauma and PTSD (pp. 192–238). Guilford Press.
Wright, F., Cooper, B.A., Conley, Y.P., Hammer, M.J., Chen, L.-M., Paul, S.M., . . . Kober, K.M. (2017). Distinct evening fatigue profiles in oncology outpatients receiving chemotherapy. Fatigue, 5(3), 131–144. https://doi.org/10.1080/21641846.2017.1322233
Wright, F., Cooper, B.A., Paul, S.M., Hammer, M.J., Conley, Y.P., Levine, J., . . . Kober, K.M. (2023). Distinct profiles of morning and evening fatigue co-occurrence in patients during chemotherapy. Nursing Research, 72(4), 259–271.
Wright, F., D’Eramo Melkus, G., Hammer, M., Schmidt, B.L., Knobf, M.T., Paul, S.M., . . . Miaskowski, C. (2015). Predictors and trajectories of morning fatigue are distinct from evening fatigue. Journal of Pain and Symptom Management, 50(2), 176–189. https://doi.org/10.1016/j.jpainsymman.2015.02.016
Wright, F., Dunn, L.B., Paul, S.M., Conley, Y.P., Levine, J.D., Hammer, M.J., . . . Kober, K.M. (2019). Morning fatigue severity profiles in oncology outpatients receiving chemotherapy. Cancer Nursing, 42(5), 355–364. https://doi.org/10.1097/NCC.0000000000000626
Wright, F., Hammer, M., Paul, S.M., Aouizerat, B.E., Kober, K.M., Conley, Y.P., . . . Miaskowski, C. (2017). Inflammatory pathway genes associated with inter-individual variability in the trajectories of morning and evening fatigue in patients receiving chemotherapy. Cytokine, 91, 187–210. https://doi.org/10.1016/j.cyto.2016.12.023
Wright, F., Kober, K.M., Cooper, B.A., Paul, S.M., Conley, Y.P., Hammer, M., . . . Miaskowski, C. (2020). Higher levels of stress and different coping strategies are associated with greater morning and evening fatigue severity in oncology patients receiving chemotherapy. Supportive Care in Cancer, 28(10), 4697–4706. https://doi.org/10.1007/s00520-020-05303-5
Xie, C., Dong, B., Wang, L., Jing, X., Wu, Y., Lin, L., & Tian, L. (2020). Mindfulness-based stress reduction can alleviate cancer-related fatigue: A meta-analysis. Journal of Psychosomatic Research, 130, 109916. https://doi.org/10.1016/j.jpsychores.2019.109916
Xu, X., Bao, H., Strait, K., Spertus, J.A., Lichtman, J.H., D’Onofrio, G., . . . Krumholz, H.M. (2015). Sex differences in perceived stress and early recovery in young and middle-aged patients with acute myocardial infarction. Circulation, 131(7), 614–623. https://doi.org/10.1161/circulationaha.114.012826
Yee, M.K., Sereika, S.M., Bender, C.M., Brufsky, A.M., Connolly, M.C., & Rosenzweig, M.Q. (2017). Symptom incidence, distress, cancer-related distress, and adherence to chemotherapy among African American women with breast cancer. Cancer, 123(11), 2061–2069. https://doi.org/10.1002/cncr.30575
Yeh, Y.-C. (2021). Symptom distress, stress, and quality of life in the first year of gynaecological cancers: A longitudinal study of women in Taiwan. European Journal of Oncology Nursing, 53, 101984. https://doi.org/10.1016/j.ejon.2021.101984
Yeun, E.J., & Jeon, M. (2020). Attitudes about coping with fatigue in patients with gastric cancer: A Q-methodology study. Gastroenterology Nursing, 43(1), 97–105. https://doi.org/10.1097/sga.0000000000000390
Zou, G., Li, Y., Xu, R., & Li, P. (2018). Resilience and positive affect contribute to lower cancer-related fatigue among Chinese patients with gastric cancer. Journal of Clinical Nursing, 27(7–8), e1412–e1418. https://doi.org/10.1111/jocn.14245




