Selection of Optimal Tobacco Cessation Medication Treatment in Patients With Cancer
Background: Tobacco use is responsible for almost half a million deaths per year in the United States, and it accounts for one-third of all cancer deaths. Limited data concerning tobacco treatment among patients with cancer are available. In addition, these patients often have complicated medical histories and are taking multiple medications. No clear, published procedures exist to help the healthcare provider select the proper medication for tobacco cessation in that context.
Objectives: This article describes the screening process established within the Tobacco Treatment Program (TTP) at the University of Texas MD Anderson Cancer Center to minimize the risk of prescribing a contraindicated tobacco cessation medication to patients with cancer. The screening process developed and used by the TTP is presented as a viable model for selecting appropriate tobacco cessation medications for patients with cancer.
Methods: The electronic medical record of each patient who uses tobacco is carefully reviewed once he or she is scheduled for a consultation. A summary is produced with a standardized template and used later as a template for the medical provider evaluation. Precautions are put in place with consideration of the characteristics of each of the tobacco cessation medications (e.g., mechanism of action, route of administration, interactions with other medications, possible side effects, contraindications). Since 2006, the TTP has had more than 4,000 new patients and more than 39,000 follow-up visits.
Findings: Because each patient with cancer has unique medical, psychological, and social circumstances, the process of selecting the optimal tobacco cessation medication needs to be individualized. Oncology healthcare providers should follow some form of screening to tailor a medication plan to each patient.
Jump to a section
Tobacco use remains one of the main causes of disease and death worldwide, accounting for at least 1 in 10 deaths among adults (World Health Organization [WHO], 2014). However, in recent years, the health hazards of using tobacco (smoked or smokeless) have become common knowledge, as have the benefits of quitting. Worldwide, the number of people who smoke has decreased since 2006, a positive trend attributable to several factors, including cigarette price increases, antismoking laws, and societal disapproval (Ng et al., 2014; WHO, 2011). However, about 44 million Americans and 1 billion people worldwide still use tobacco. The percentage of American adults who smoke has declined since 2005, reaching a plateau of about 18%, but this means that about 1 in 5 adults still smoke (Agaku, King, & Dube, 2014). In the United States, smoking is more prevalent among men, American Indians and Alaska Natives, and people with mental illness, without higher education, and who live below the poverty level (Centers for Disease Control and Prevention [CDC], 2013).
Tobacco use has been associated with serious health problems (e.g., cancer, coronary artery disease, cerebrovascular disease, chronic obstructive pulmonary disease, arthritis) (U.S. Department of Health and Human Services [USDHHS], 2014). In the United States, tobacco use is responsible for about 1 in 5 deaths annually (USDHHS, 2014). Smoking also is a known risk factor for 17 types of cancers, and research has shown that it negatively affects treatment and prognosis (Burke, Miller, Saad, & Abraham, 2009; CDC, 2014). Fewer than half of all smokers quit smoking after being diagnosed with cancer, and only 62% of these smokers report having been educated by their doctors about tobacco cessation (Burke et al., 2009). These figures underscore the importance of providing patients with cancer with tobacco cessation counseling, medications, and encouragement to achieve abstinence from smoking or the use of smokeless tobacco. For this reason, well-developed and structured programs focused on helping patients with cancer achieve this goal are needed. Following the clinical practice guideline recommendations for treating tobacco use and dependency, such programs would deliver practical counseling and support, as well as effective tobacco cessation medications. Seven first-line medications have been approved by the U.S. Food and Drug Administration (FDA) and are shown to increase smoking abstinence rates by twice as high as the placebo; they are bupropion, nicotine gum, nicotine inhalers, nicotine lozenges, nicotine nasal sprays, nicotine patches, and varenicline (USDHHS, 2008).
The University of Texas MD Anderson Cancer Center in Houston has a comprehensive, well-established tobacco treatment program. Its mission is to implement a tobacco cessation and relapse-prevention program for its patients with cancer and employees. The center’s Tobacco Treatment Program (TTP) is funded by the institution through tobacco settlement funds to the state of Texas. In 2006, the TTP began offering free tobacco cessation counseling and medications to patients with cancer and center employees through a multidisciplinary team of social workers, psychologists, and support staff, as well as a psychiatrist, physician assistant, and RN. The TTP offers participants six to eight in-person or telephone counseling sessions based on problem-solving techniques, motivational interviewing, and cognitive behavioral strategies. The program also offers long-term telephone follow-up for as long as 12 months following the end of treatment.
Program participants included patients at the MD Anderson Cancer Center who were referred to the program by a physician or other healthcare provider, were self-referred, or identified themselves as smokers or recent quitters (within the year) in an electronic needs assessment questionnaire, which is completed by all patients during admission and every three months thereafter. The program extends to patients’ significant others who live in the same household and whose smoking is affecting the patient’s ability to quit. The MD Anderson Cancer Center employees, as well as their spouses and dependents, are also eligible to participate in the TTP and can self-refer.
Methods
This article presents a model for selecting medications that is based on the current authors’ experience with the TTP, a program that had more than 4,000 new patients and more than 39,000 follow-up visits from January 2006 to October 2013. In 2011, the authors analyzed the six-month follow-up data on the basis of cohorts treated from the start of the program in 2006 until the end of 2010. The six-month abstinence rate (seven-day point prevalence at six months after the end of treatment) among those who were able to reach abstinence (respondent only) was 46% (n = 1,291, response rate = 74%). However, when an intention-to-treat model is used (including all patients treated at baseline and assuming all those lost to follow-up have relapsed to smoking), the six-month abstinence rate (seven-day point prevalence at six months after the end of treatment) dropped to 34% (n = 1,670). Pharmacologic interventions are provided free of charge in the TTP for as long as 10 weeks. The medications prescribed by TTP medical staff and recommended in the clinical practice guidelines for treating tobacco use and dependency include nicotine replacement therapy (NRT), varenicline, and bupropion, or combinations of NRTs or NRTs with bupropion. If participants succeed at quitting, they are encouraged to remain on medications (bupropion or varenicline) for an additional three months because of evidence of improved efficacy of these medications when used for as long as six months (Hays et al., 2001; Tonstad et al., 2006).
Oral and Nasal Nicotine Replacement Therapies
NRTs supply low doses of nicotine, but they do not contain the toxins found in tobacco smoke. These products include nicotine patches, gum, lozenges or mini-lozenges, inhalers, and nasal sprays. They are believed to work on nicotine receptors as agonists. The systemic absorption rate of each of the different nicotine replacement products varies. For example, with oral administration (nicotine gum and lozenges), the systemic absorption rate is slower than when nicotine is administered via the inhaled or intranasal route, but faster than the topical route (nicotine patch). Still, cigarette smoking has the fastest absorption rate; nicotine reaches the brain within 7–10 seconds of starting to smoke a cigarette. Commonly reported potential side effects of NRTs include skin irritation at the site of application, pruritus, and sleep disturbance (nicotine patch); dyspepsia and hiccups (nicotine gum and lozenge); throat irritation (nicotine inhaler); and mucosa irritation and a burning sensation (nicotine nasal spray).
Varenicline
Approved by the FDA in May 2006, varenicline is a potent and competitive partial agonist at the alpha4/beta2 neuronal nicotinic acetylcholine receptors. It provides some nicotine-like effects to ease nicotine withdrawal symptoms. In addition, by binding strongly to those nicotinic receptors, it blocks the effects of nicotine from tobacco use, attenuating its effect on the brain. The most common side effects of varenicline are nausea, vivid dreams, constipation, and flatulence. Several serious neuropsychiatric side effects have been associated with varenicline, including depression, coordination difficulties, decrease in concentration, and, more rarely, increased aggressiveness and suicidal ideation (Physicians’ Desk Reference, 2011). However, to date, a causal relationship has not been demonstrated between the use of varenicline and these symptoms, particularly because nicotine dependence and withdrawal syndrome are known to be linked to suicidal ideation, negative affectivity, and depressive symptoms (Jiménez-Ruiz, Berlin, & Hering, 2009; Tonstad, Davies, Flammer, Russ, & Hughes, 2010).
In addition, reports on varenicline’s potential to cause cardiovascular events have been conflicting. In June 2011, the FDA issued a public advisory highlighting the need for caution when prescribing varenicline to patients with cardiac disease. This report was followed by two publications: a meta-analysis (Prochaska & Hilton, 2012) and the latest FDA (2013) report. Neither showed a statistically significant difference in the occurrence of cardiovascular events between varenicline and placebo groups.
Bupropion
Bupropion is a selective inhibitor of the neuronal reuptake of noradrenaline and dopamine that was initially approved for the treatment of depression, then for tobacco cessation in the United States in 1996 (Physicians’ Desk Reference, 2011). Bupropion’s mechanism of action in tobacco cessation is not well understood; however, bupropion is thought to be a nicotine receptor blocker (Damaj et al., 2004). The most common side effects of bupropion are dry mouth and insomnia, but neuropsychiatric side effects (e.g., depression, irritability, suicidal ideation) have also been reported, but they are rare. A gradual increase from 150 mg per day for three days to 300 mg per day helps to minimize the side effects (Physicians’ Desk Reference, 2011).
Other tobacco cessation medications are considered in the TTP when the commonly prescribed agents do not provide the desired effect. These so-called second-line tobacco treatment medications, such as clonidine and nortriptyline, are used less often and do not have an official FDA indication for tobacco cessation. Other medications, such as topiramate, have recently been proposed for off-label use. The use of topiramate for tobacco cessation is based on prior studies of topiramate for treatment of alcohol dependence in which added benefits for tobacco cessation were reported (Johnson et al., 2007).
This article describes the MD Anderson Cancer Center’s TTP method for screening patients with cancer before placing them on any medications for tobacco cessation. The goal is to avoid contraindications and drug interactions and to develop the safest tailored medication plan to help participants achieve tobacco cessation and abstinence.
Program-Specific Procedures
Prospective TTP participants are referred to the program by their primary oncologist or other healthcare professionals involved in their care, or through self-referral via an electronic needs assessment questionnaire. Once they are identified, they are contacted by program staff and offered an appointment. All contact attempts are documented in a database program developed for that purpose. Before a patient’s initial consultation, the program’s RN reviews electronic medical records of all scheduled new patients; these records of each patient’s medical history include current or recent laboratory results that may be abnormal. The RN then summarizes the patient’s history in a standardized template that prescribing clinicians use as the basis for the medical evaluation on the day of the consultation (see Figure 1). This constitutes the first step in the process of determining an appropriate and safe tobacco cessation medication therapy. When screening patients for a first-line tobacco cessation medication, the considerations are NRTs, varenicline, and bupropion. 
Nicotine Replacement Therapies
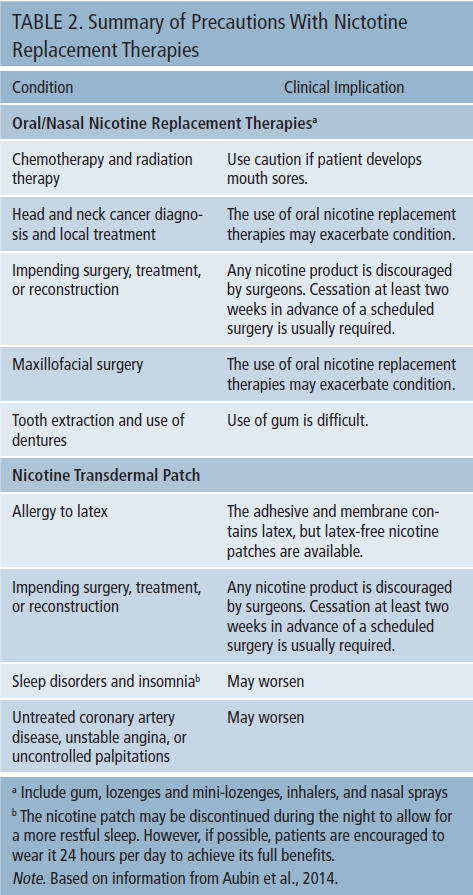
Oral and nasal NRTs are available in several forms, including nicotine lozenges and mini-lozenges, gum, inhalers, and nasal sprays. Nicotine lozenges are available in regular and miniature sizes and in 2 mg and 4 mg strength. Nicotine inhalers come in cartridges containing 10 mg of nicotine, whereas nicotine nasal sprays are packaged in a kit with four bottles, each containing 10 mg/ml of nicotine. The nicotine transdermal patch comes in latex and latex-free forms. Both are available in three dosages: 7 mg, 14 mg, and 21 mg. Dosing regimens and specific use information on NRTs is summarized in Table 1. Table 2 presents various conditions and factors to which attention should be paid before recommending NCTs. 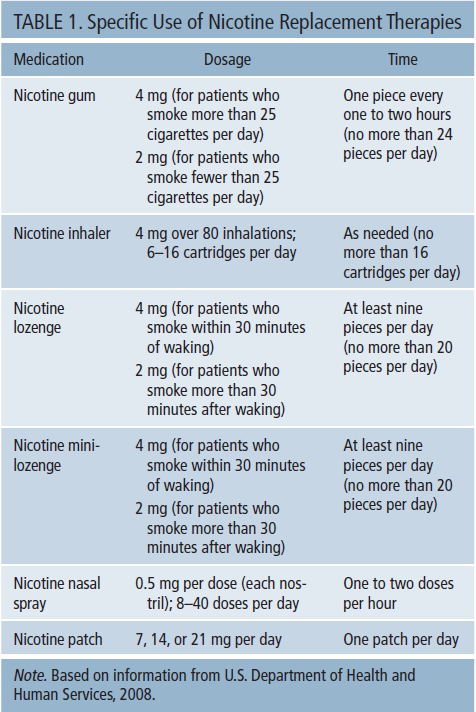
Varenicline
This medication is prescribed and administered in a run-up dosing regimen; this gradual increase of varenicline helps to minimize possible side effects. The typical dose is 0.5 mg in the morning (after a meal) for three days and then twice a day for four days, followed by the higher dose of 1 mg twice a day thereafter. Varenicline is cleared mostly by the kidneys; attention is paid to serum creatinine levels, and a creatinine clearance (CC) value is calculated. For a patient to be on the full dose (2 mg per day), the CC value needs to be equal to or higher than 70 mg/dl. If the creatinine clearance is between 30–50 mg/dl, the dose may be reduced. If the CC value is less than 30 mg/dl, varenicline is not provided unless it is the only option. In this situation, a modified, very low dose of varenicline 0.25–0.5 mg per day could be used under close observation. Varenicline should be stopped if side effects develop (Physicians’ Desk Reference, 2011) (see Table 3). 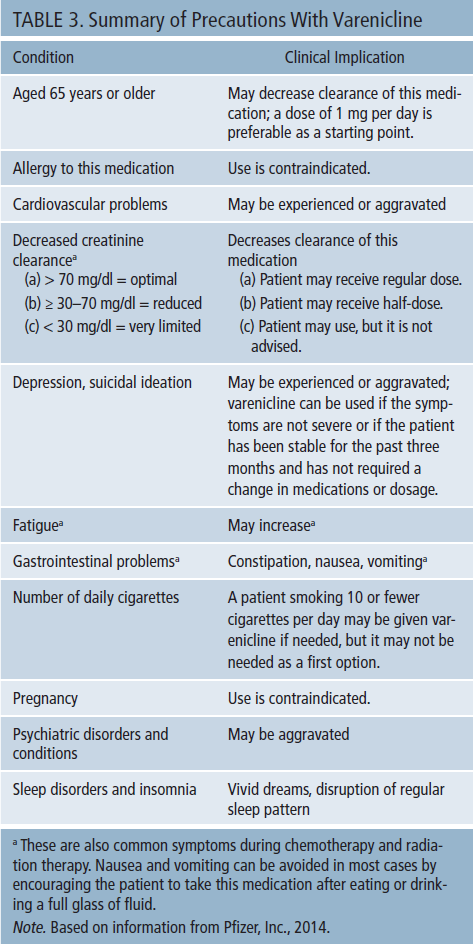
Bupropion
Different forms of bupropion are available (i.e., immediate release, slow release [SR], and extended release [XL]). The TTP favors the XL formulation because (a) it is taken once a day, which patients find facilitates easier adherence, and (b) it has been reported to cause fewer side effects (it has a slower uptake and decline in blood levels). The TTP’s usual run-up dose for any of the bupropion formulations for tobacco cessation is 150 mg per day for seven days, then 300 mg per day thereafter. Although the FDA has approved bupropion to be used for 150 mg per day for three days, then increased to 300 mg per day on the fourth day (Physicians’ Desk Reference, 2011), the authors have found it to be too rapid of an increase that tends to precipitate unnecessary side effects (e.g., insomnia, tremor, irritability, agitation). The authors’ preference is to wait at least one week before increasing to 300 mg per day.
Special attention should be paid to a history of seizures or conditions that increase the risk of seizures (e.g., head trauma, cerebrovascular accidents). In addition, liver function tests should be monitored to determine that values are not too elevated (no more than 2.5 times the upper limit) because bupropion is metabolized in the liver, and its metabolites can accumulate in a toxic fashion. Other significant conditions and factors are summarized in Table 4. 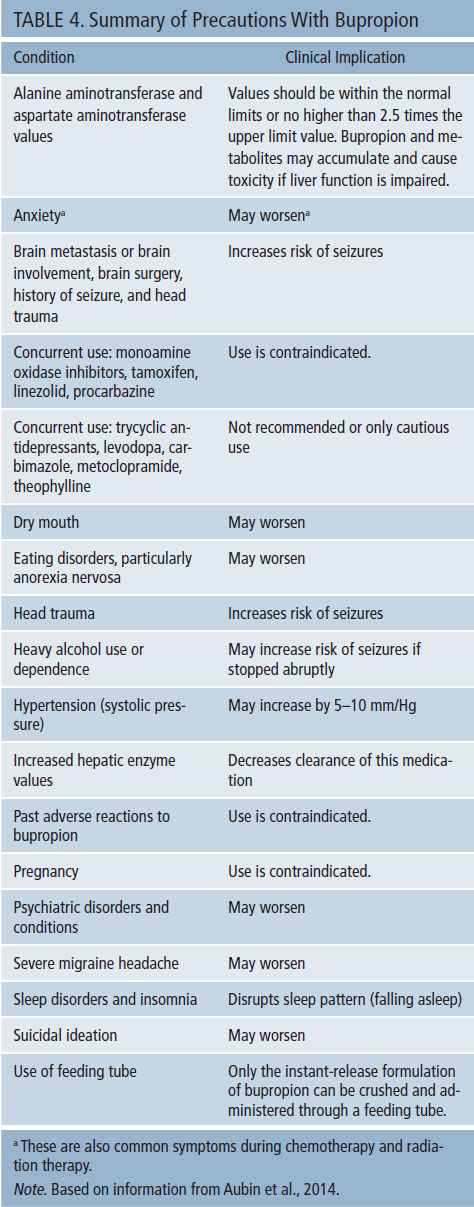
Additional Considerations
All patients receiving therapy with warfarin, insulin, oral hypoglycemic agents, or theophylline, independently of the tobacco cessation pharmacotherapy choice, should be educated about possible changes in the metabolism of these medications once tobacco is no longer used. For example, tobacco cessation may reduce the clearance of warfarin, making it extremely important for patients to closely monitor their coagulation values. Similarly, patients who are on theophylline should monitor their theophylline levels after tobacco cessation. In the case of insulin, tobacco smoking is known to increase insulin resistance. Therefore, the cessation of tobacco use may result in a decrease in the levels of glucose in the blood. The same holds true for most oral hypoglycemic agents. In such instances, patients should be made aware of these possible changes and counseled to notify their primary healthcare providers about their involvement with tobacco cessation. They should also be advised to keep all appointments with the warfarin clinic (in the case of their use of this medication) and closely monitor their blood sugar levels (in the case of their use of insulin and oral hypoglycemic agents) (Physicians’ Desk Reference, 2011).
Discussion
The process of tailoring the tobacco cessation medication plan to each patient is not, and should not be, a random one. Each patient is unique and has unique circumstances that will have implications for the choice of the most appropriate pharmacotherapy among the ones currently approved by the FDA for tobacco cessation. This is true for all patients but particularly for patients with cancer, whose lives have been reshaped and modified by innumerable factors. These factors may affect their abilities at all levels (e.g., psychological, physiologic, cognitive, family dynamics).
The implications for nurses are significant. Any healthcare provider and his or her multidisciplinary team would need to take into account all of these factors when considering the different tobacco cessation medications available. Providers should take a meticulous and careful approach to screening patients for the optimal pharmacologic treatment for tobacco use—one that will have the best potential for success and the least possibility of causing any harm or undesirable side effects. Although the primary aim of this article is to encourage and recommend a thorough approach, the authors also want to strike the right tone in being clear that first-line tobacco cessation medications are, in general, safe. Unfortunately, many of these medications have received undue scrutiny in the literature and the media, particularly when one considers the known risk-benefit ratio of taking them versus continuing to smoke. One clear example is the original instruction that warns of a high risk for cardiovascular events while using NRTs and continuing to smoke. The FDA is now working to remove this warning from the package inserts of these medications (Koch, 2013). That high risk for cardiovascular events was proven not to be true; in fact, NRTs are now recommended for use before quitting tobacco (Campaign for Tobacco-Free Kids, 2013). Another example of excessive precautions is the perception of bupropion as having a high risk of inducing seizures. In reality, that risk on the commonly used doses of bupropion (150 or 300 mg per day) is less than or equal to the risk of seizure while on other antidepressants (Hughes, Stead, Hartmann-Boyce, Cahill, & Lancaster, 2014). In addition, the recent association of varenicline with cardiovascular events is another example of inaccuracy that so far has not been proven to be the case. The increase in neuropsychiatric events may or may not hold to be true in association with varenicline; knowing that close monitoring is the best strategy is important. If any symptoms develop, one can suspend the medication and, within one day, one-half of the amount of varenicline in the body would be eliminated. Finally, the American Cancer Society website (www.cancer.org) has trustworthy information about tobacco cessation and the process of quitting that patients can use to their advantage.
Conclusion
Selecting the most appropriate tobacco cessation medication for patients with cancer should be done carefully and, ideally, in a structured manner. Treatment plans should be tailored to individual patients, taking into account each patient’s needs and preferences. Doing so will maximize the chances of a successful outcome.
The authors gratefully acknowledge the Department of Scientific Publication at the University of Texas MD Anderson Cancer Center for reviewing and editing the manuscript.

References
Agaku, I.T., King, B.A., & Dube, S.R. (2014). Current cigarette smoking among adults—United States, 2005–2012. Morbidity and Mortality Weekly Report, 63, 29–34.
Aubin, H.J., Luquiens, A., & Berlin, I. (2014). Pharmacotherapy for smoking cessation: Pharmacological principles and clinical practice. British Journal of Clinical Pharmacology, 77, 324–336. doi:10.1111/bcp.12116
Burke, L., Miller, L.A., Saad, A., & Abraham, J. (2009). Smoking behaviors among cancer survivors: An observational clinical study. Journal of Oncology Practice, 5, 6–9. doi:10.1200/jop.0912001
Campaign for Tobacco-Free Kids. (2013). FDA takes welcome steps to help more smokers quit: Statement of Matthew L. Myers, president, Campaign for Tobacco-Free Kids. Retrieved from http://www.tobaccofreekids.org/press_releases/post/2013_04_01_fda
Centers for Disease Control and Prevention. (2013). Adult smoking: Focusing on people with mental illness. Retrieved from http://www.cdc.gov/vitalsigns/smokingandmentalillness/index.html
Centers for Disease Control and Prevention. (2014). Smoking and cancer. Retrieved from http://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/pdfs/fs…
Damaj, M.I., Carroll, F.I., Eaton, J.B., Navarro, H.A., Blough, B.E., Mirza, S., . . . Martin, B. (2004). Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Molecular Pharmacology, 66, 675–682. doi:10.1124/mol.104.001313
Hays, J.T., Hurt, R.D., Rigotti, N.A., Niaura, R., Gonzales, D., Durcan, M.J., . . . White, J.D. (2001). Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. A randomized, controlled trial. Annals of Internal Medicine, 135, 423–433. doi:10.7326/0003-4819-135-6-200109180-00011
Hughes, J.R., Stead, L.F., Hartmann-Boyce, J., Cahill, K., & Lancaster, T. (2014). Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews, 8, CD000031. doi:10.1002/14651858.CD000031.pub4
Jiménez-Ruiz, C., Berlin, I., & Hering, T. (2009). Varenicline: A novel pharmacotherapy for smoking cessation. Drugs, 69, 1319–1338.
Johnson, B.A., Rosenthal, N., Capece, J.A., Wiegand, F., Mao, L., Beyers, K., . . . Swift, R.M. (2007). Topiramate for treating alcohol dependence: A randomized controlled trial. JAMA, 298, 1641–1651. doi:10.1001/jama.298.14.1641
Koch, W. (2013, September 4). Big tobacco back in TV viewers’ faces as FDA cuts in. Retrieved from http://www.usatoday.com/story/news/nation/2013/09/03/big-tobacco-tv-eci…
Ng, M., Freeman, M.K., Fleming, T.D., Robinson, M., Dwyer-Lindgren, L., Thomson, B., . . . Gakidou, E. (2014). Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA, 311, 183–192. doi:10.1001/jama.2013.284692
Pfizer, Inc. (2014). Chantix® (varenicline) tablets: Highlights of prescribing information. Retrieved from http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021928s032s036…
Physicians’ Desk Reference (66th ed.). (2011). Montvale, NJ: Thomson PDR.
Prochaska, J.J., & Hilton, J.F. (2012). Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: Systematic review and meta-analysis. BMJ, 344, e2856. doi:10.1136/bmj.e2856
Tonstad, S., Davies, S., Flammer, M., Russ, C., & Hughes, J. (2010). Psychiatric adverse events in randomized, double-blind, placebo-controlled clinical trials of varenicline: A pooled analysis. Drug Safety, 33, 289–301. doi:10.2165/11319180-000000000-00000
Tonstad, S., Tønnesen, P., Hajek, P., Williams, K.E., Billing, C.B., & Reeves, K.R. (2006). Effect of maintenance therapy with varenicline on smoking cessation: A randomized controlled trial. JAMA, 296, 64–71. doi:10.1001/jama.296.1.64
U.S. Department of Health and Human Services. (2008). Treating tobacco use and dependence: 2008 update. Retrieved from http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recom…
U.S. Department of Health and Human Services. (2014). The health consequences of smoking—50 years of progress: A report of the surgeon general. Retrieved from http://www.surgeongeneral.gov/library/reports/50-years-of-progress/full…
U.S. Food and Drug Administration. (2013). FDA drug safety communication: Safety review update of Chantix (varenicline) and risk of cardiovascular adverse events. Retrieved from http://www.fda.gov/Drugs/DrugSafety/ucm330367.htm
World Health Organization. (2011). WHO report on the global tobacco epidemic, 2011: Warning about the dangers of tobacco: Executive summary. Retrieved from http://www.who.int/tobacco/global_report/2011/exec_summary/en
World Health Organization. (2014). Tobacco. Retrieved from http://www.who.int/mediacentre/factsheets/fs339/en/index.html
About the Author(s)
Rosario Wippold, RN-MPH, is a research nurse, Maher Karam-Hage, MD, and Janice Blalock, PhD, are associate professors in the Department of Behavioral Science, and Paul Cinciripini, PhD, is director of the Tobacco Treatment Program and chair ad interim of the Department of Behavioral Science, all at the University of Texas MD Anderson Cancer Center in Houston. The authors take full responsibility for the content of the article. This study was supported, in part, by a grant (No. P30 CA16672) from the National Institutes of Health. The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers or editorial staff. Karam-Hage can be reached at maherkaram@mdanderson.org, with copy to editor at CJONEditor@ons.org. (Submitted November 2013. Revision submitted June 2014. Accepted for publication June 22, 2014.)

