Immune Checkpoint Inhibitor Therapy: Key Principles When Educating Patients
Background: Immune checkpoint inhibitor (ICI) therapy is a fast-developing field within the spectrum of cancer care. ICIs are associated with distinctive immune-related adverse events (irAEs), reflecting their unique mechanisms of action.
Objectives: Effective management of irAEs requires early recognition and prompt reporting of their signs and symptoms; appropriate patient education is critical to maximizing this opportunity.
Methods: A comprehensive literature search was conducted in the public domain concerning awareness, assessment, and management of irAEs associated with ICIs.
Findings: Educational resources should provide timely, consistent, and personalized information, using a variety of teaching strategies that consider individual patient needs. Patient education should be developed with interprofessional team input and regularly reviewed in response to emerging guidance. Key messages include timing of therapeutic response and corresponding irAEs, early identification of irAEs, and the unique ability of ICIs to influence immune responses after treatment discontinuation.
Jump to a section
Immune checkpoint inhibitors (ICIs), a class of immunotherapy that works by restoring immune system function, have been increasingly used in clinical practice (Haanen, Thienen, & Blank, 2015; Kottschade et al., 2016; Postow, Callahan, & Wolchok, 2015). The U.S. Food and Drug Administration (FDA) approved ipilimumab to treat metastatic melanoma in 2011 and, since then, several ICIs have been approved to treat a range of cancers, including melanoma, Merkel cell carcinoma, Hodgkin lymphoma, and non-small cell and small cell lung, kidney, head and neck, colorectal, liver, bladder, cervical, and microsatellite instability high or mismatch repair deficient cancers (marking the first approval based on a biomarker rather than tumor type) (AstraZeneca, 2018; Bristol-Myers Squibb, 2018a, 2018b; FDA, 2018; Genentech, 2018; Merck & Co., 2018; Pfizer, Inc., 2017).
The aim of ICI therapy is to enhance the body’s anti-tumor immunity by disrupting the pathways that tumors use to evade the immune system (Beatty & Gladney, 2015; Kannan, Madden, & Andrews, 2014). In normal tissue, immune checkpoint pathways, including cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1), downregulate T-cell activity to prevent autoimmunity (de Mello, Veloso, Esrom Catarina, Nadine, & Antoniou, 2016; Haanen et al., 2015). Some tumors aberrantly upregulate these pathways to avoid immune surveillance (Dougan & Dranoff, 2009; Kannan et al., 2014). Blocking tumor inhibitory pathways with ICIs can induce an anti-tumor immune response (de Mello et al., 2016) (see Figure 1). 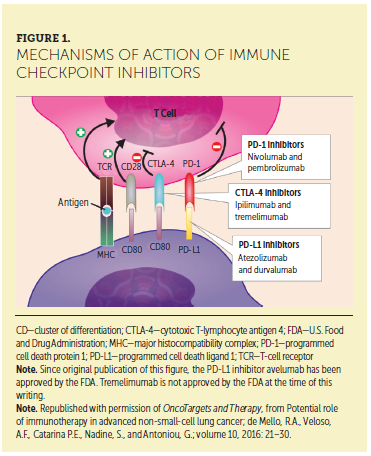
Because immune checkpoints help to balance the immune system, ICIs are associated with immune-related adverse events (irAEs), reflecting their immune-based mechanisms (Haanen et al., 2015; Weber, Postow, Lao, & Schadendorf, 2016). irAEs are commonly dermatologic, gastrointestinal, hepatic, endocrine, or respiratory, but can affect other organ systems (Haanen et al., 2017; Weber et al., 2016). Most are mild to moderate in severity, although serious and occasionally life-threatening irAEs have been reported (Kwon et al., 2014; Puzanov et al., 2017). Although serious irAEs may result in treatment discontinuation, with prompt recognition and management, most resolve and patients can continue ICI treatment (Brahmer et al., 2018; Haanen et al., 2017; Puzanov et al., 2017; Weber et al., 2016). Patient education is critical to maximize early recognition and reporting of irAEs (Fradkin, 2017).
Although multiple patient ICI education resources are available, little has been published regarding best practices for informing patients and caregivers about ICIs. In 2017, the Cancer Support Community conducted a needs assessment of 95 patients receiving ICIs and 45 of their caregivers (Aspiras, Power, & Gonzalo, 2018). Most patients (55%) and caregivers (85%) identified “understanding how to cope with ICI symptoms and side effects” as important, while many patients (50%) and caregivers (73%) reported difficulty obtaining this information (Aspiras et al., 2018).
To address these and other educational needs, a comprehensive literature search of irAE management practices and patient irAE education resources was performed, and included publications from PubMed, congress abstracts, professional and patient advocacy groups, practice guidelines, and ICI prescribing information. This review summarizes patient education regarding chemotherapy, shares information about best practices, and identifies barriers to patient education about ICIs, as well as provides information about patient education methods and support tools. To provide appropriate and effective education to patients and caregivers, nurses must become knowledgeable about immunotherapy and irAEs (Aim With Immunotherapy Foundation, 2019; Dine, Gordon, Shames, Kasler, & Barton-Burke, 2017; Kannan et al., 2014; Kottschade et al., 2016).
Patient Education
Experience based on chemotherapy education shows that education is essential for patients with cancer to understand how to best care for themselves by managing treatment side effects and contacting healthcare providers (HCPs) for assistance (Valenti, 2014). Effective patient education during the initial diagnosis and treatment can improve anxiety and self-care decisions, decrease side effects, and enhance quality of life (Shahsavari, Matory, Zare, Taleghani, & Kaji, 2015; Tian, Lia, & Cheng, 2015).
Patient education is most effective when provided before treatment initiation, in a quiet environment that supports learning, and by oncology team members in the primary oncology setting (Garcia, 2014). Treatment side effects, management strategies, and infusion center orientation are consistently shown as the most important topics to help reduce patient anxiety (Garcia, 2014). Education structure is an important consideration to maximize information retention. However, a review of teaching methods (e.g., audio or video recording, in-person class, one-on-one discussion, telephone calls) reported no single method to be significantly more effective than any other in delivering information to patients because knowledge retention is largely based on individual learning preferences (Valenti, 2014).
Patient use of different teaching resources is associated with different factors, including sociodemographics (e.g., age, gender, education), psychosocial characteristics (e.g., stress level, coping strategies), unfulfilled information needs, and perceived credibility of information sources (Mekuria, Erku, & Belachew, 2016). For example, education through audio or video recordings may benefit patients with literacy issues (Valenti, 2014). Before planning educational activities, oncology nurses can conduct a learning needs assessment with individual patients to determine factors such as health literacy level, reading skills, personal preferences for learning, cultural or religious aspects, pain level, and amount of anxiety (Canadian Partnership Against Cancer, 2018; Goldsmith & Terui, 2018; Marcus, 2014). Although no single needs assessment is recommended, examples of wider needs assessment tools incorporating educational elements were developed and reviewed (Australian Cancer Survivorship Centre, 2016). Involving patients in resource selection can help ensure that patients’ needs and preferences are addressed, improving acceptance and effectiveness (Jewitt et al., 2016).
Although initial education is essential, it is important to continually reinforce teaching, particularly when irAE occurrence results in treatment plan changes. The teach-back method, which allows patients to repeat what they learned to the HCP, enabling the HCP to determine which information was confusing or not understood, is useful for assessing patient comprehension and understanding (Portz & Johnston, 2014). Patients should understand the goals and importance of activities they take part in, and they should be encouraged to ask their oncology team any questions they have before and after teaching and throughout the treatment process (Valenti, 2014). Patients value the expertise of oncology nurses and group support from others receiving treatment (Fee-Schroeder et al., 2013).
Best-Practice Sharing in Patient Education
The experiences of teaching about the early stages of ICI development, such as ipilimumab in advanced melanoma, suggest that early recognition of symptoms and frequent monitoring are central to irAE management; patients and caregivers should be educated about signs and symptoms to monitor and report (Fecher, Agarwala, Hodi, & Weber, 2013). Patients may not accurately report side effects for fear of being taken off treatment; therefore, patients should be encouraged to “call early and call often” to discuss any changes in health status with their cancer team (Fecher et al., 2013). Patients should be taught that irAEs can often be managed effectively without permanent treatment discontinuation, particularly when identified early (Bayer et al., 2017; Brahmer et al., 2018).
ICI-related toxicity patterns based on tumor type are not well established, and evidence for which patients are at elevated risk remains unclear (Brahmer et al., 2018). Patients with preexisting autoimmune conditions may be at risk of underlying disease recurrence and require close monitoring; therefore, effective ICI education for patients with preexisting autoimmune disease or prior organ transplantation requires a thoughtful discussion of potential risks and benefits (Brahmer et al., 2018).
Barriers to Patient Education
As ICIs continue to evolve with increasing availability and new combinations, nurses can provide patients and caregivers with timely ICI education, including mechanisms of action and clinical profiles of irAEs (Brahmer et al., 2018). However, in this fast-developing field, information can become antiquated quickly. For example, the nature and range of irAEs are becoming more clearly defined with increasing patient exposure to ICIs, and new rare side effects, such as meningoradiculitis, polyradiculitis, cardiac arrhythmia, asystolia, aphasia, paresis, and Parkinson syndrome, have been reported (Puzanov et al., 2017; Zimmer et al., 2016). Cancer centers can obtain new data relating to safety and side effect management of ICI treatment, as well as regularly review and update ICI patient education materials and activities, to ensure that clinical staff are equipped to deliver ICI treatment.
Organizing high-quality patient education initiatives takes time and commitment by nurses, doctors, pharmacists, psychologists, and administrative staff (Cipolat Mis et al., 2015; Fradkin, 2017). Education covering irAE management is complex, requiring message consistency and an interprofessional approach involving oncologists and internal medicine specialists (e.g., endocrinologists, gastroenterologists, pulmonologists) (Abdel-Wahab, Alshawa, & Suarez-Almazor, 2017; Fay, Moreira, Nunes Filho, Albuquerque, & Barrios, 2016). Oncology nurses have frequent contact with patients and other staff members and play a critical role in ensuring that education initiatives are patient-centered and collaborative (Fay et al., 2016; Garcia, 2014).
Patient Education for Immune Checkpoint Inhibitors
Guidelines on the management of ICI-associated irAEs were published by the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) (Brahmer et al., 2018), the Society for Immunotherapy of Cancer (SITC) (Puzanov et al., 2017), and the European Society for Medical Oncology (ESMO) (Haanen et al., 2017). Guidelines recommend providing ICI-specific information to patients and caregivers before initiating therapy (Brahmer et al., 2018; Haanen et al., 2017; Puzanov et al., 2017). ASCO–NCCN guidelines also recommend continued patient and caregiver education throughout treatment and survivorship (Brahmer et al., 2018).
ICIs are associated with a spectrum of AEs that differ from other systemic therapies. Patients should be informed that ICIs work differently than chemotherapy or targeted therapies that patients may have received previously, producing unique therapeutic responses and corresponding irAEs (Brahmer et al., 2018). In particular, irAEs can have delayed onset and prolonged duration compared with chemotherapy-related AEs (Puzanov et al., 2017).
Patients may present with irAEs late in ICI treatment or even months after treatment discontinuation (Haanen et al., 2017; Puzanov et al., 2017). At any point, effective management depends on early recognition of irAEs and prompt intervention (Puzanov et al., 2017). Therefore, patients are encouraged to report changes to their providers (Brahmer et al., 2018).
Educating Patients and Caregivers
The following section provides key messages to be communicated to patients and caregivers regarding what to expect from ICI treatment, with supporting information to provide additional context for HCPs.
Background and mechanism of action: ICIs work with the immune system to fight cancer. This can result in unique response patterns and side effects that differ from the cancer treatments patients may have previously received (such as chemotherapy). Tumors can avoid immune system detection through upregulating pathways that limit immune responses (Beatty & Gladney, 2015; Kannan et al., 2014). ICIs block these pathways, restoring balance to a patient’s immune response by allowing T cells to work against cancer cells (de Mello et al., 2016; Dougan & Dranoff, 2009; Kannan et al., 2014). Currently approved ICIs include antibodies that block specific immune checkpoint molecules, such as CTLA-4, PD-1, and PD-L1 (a ligand of PD-1) (Michot et al., 2016; Spain, Diem, & Larkin, 2016).
Expected response to treatment: ICIs can result in long-term responses and survival, even if the initial response is delayed or if progression is experienced before the response to treatment (Michot et al., 2016). Immune responses to therapy can vary between patients and from typical response patterns seen with chemotherapy or radiotherapy (Fay et al., 2016; Kannan et al., 2014). This is because immune responses can occur months after treatment, even after an initial increase in tumor size or appearance of new tumors (Wolchok et al., 2009).
Monitoring and management of side effects: Patients should inform the cancer team immediately if any changes are noticed. Most side effects of ICIs can be managed if treated early, and stopping ICIs is not always necessary. Depending on how serious the side effects are, patients may receive steroids and/or delay further ICIs until they improve. Prompt reporting of potential side effects is key to effective management and completion of ICIs. A thorough baseline symptom assessment before starting ICIs is important to better identify possible treatment-emergent changes (Lewis, 2016). Such discussions also facilitate patient and caregiver education about potential symptoms or symptom changes to look for. Side effects of ICIs often involve inflammation of the skin, stomach, lung, liver, and endocrine system, but can potentially affect any system (Kannan et al., 2014; Kottschade et al., 2016; Michot et al., 2016). Some patients experience fatigue, which may be a standalone symptom or an indication of immune-related endocrinopathies (potentially secondary to hypophysitis) (Puzanov et al., 2017). Table 1 provides information for patients and HCPs on signs and symptoms of common irAEs and appropriate management. Although these side effects are usually mild to moderate and manageable, they can become severe or even life-threatening if not identified and managed quickly (Kannan et al., 2014; Kottschade et al., 2016; Michot et al., 2016). Appropriate management depends on location and severity, often involving temporary treatment to suppress immune system overactivation (Puzanov et al., 2017). Tumor responses have been seen in patients who discontinued therapy because of AEs (Schadendorf et al., 2017). 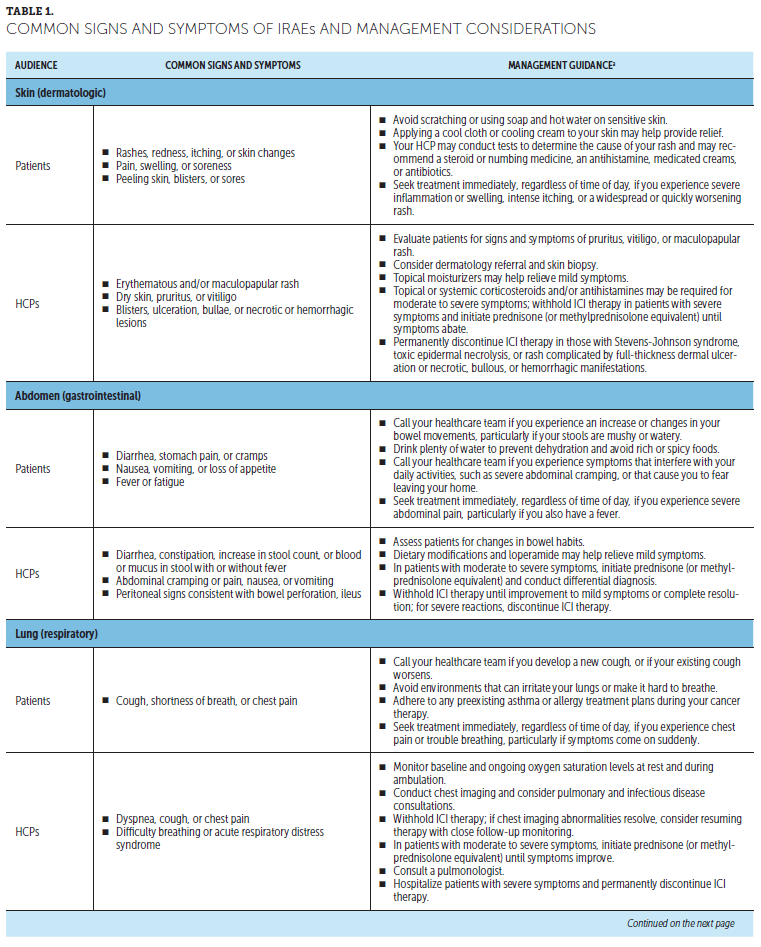
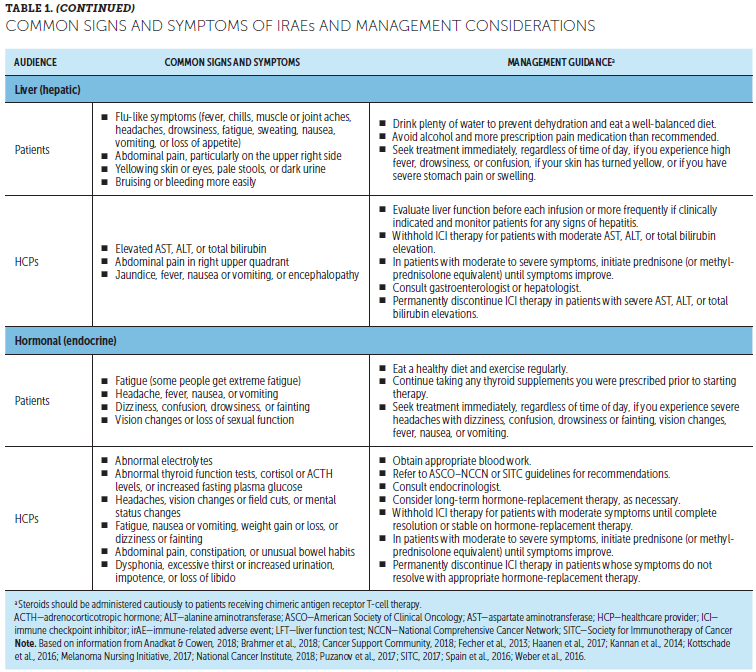
When to expect side effects: Side effects of ICIs can occur at any time, including as early as the day after the first treatment infusion or even after ending treatment. ICIs can influence a patient’s immune response even after treatment has ended (Brahmer et al., 2018; Haanen et al., 2017; Puzanov et al., 2017). Most side effects occur within three months of starting ICIs but can also develop as long as a year after ending treatment (Haanen et al., 2017).
When to contact the cancer team and other healthcare providers: Patients should quickly alert the cancer team and other HCPs if they experience potential side effects of ICIs or if any symptoms change or worsen. Because side effects can occur even after therapy has ended, patients should stay in contact with their cancer team and tell other HCPs (e.g., general practitioners, emergency department physicians) that they are receiving or have received ICIs (Kannan et al., 2014). This is important because patients often see multiple HCPs, who should be aware of the potential for irAEs (Brahmer et al., 2018).
Patient Education Methods and Support Tools
ICI-specific patient and caregiver educational materials are in relative infancy, but the importance of providing unique ICI education and support is increasingly recognized. Stakeholders, ranging from the pharmaceutical industry, professional societies, and cancer programs to patient advocacy and support groups, are developing ICI-related patient education and support resources, including print and online materials, events, and workshops (Association of Community Cancer Centers, 2016).
Although HCPs are still the most highly trusted source for information about cancer and cancer treatment, more than half of patients seek additional related information online (George et al., 2018; Shea-Budgell, Kostaras, Myhill, & Hagen, 2014). Online ICI resources for patients are available from various organizations, including general cancer care and patient advocacy and support groups, tumor-specific organizations, hospitals and cancer centers, professional organizations and societies, and pharmaceutical companies (Association of Community Cancer Centers, 2016) (see Table 2). These typically include information on how ICIs work, different cancer and treatment types, treatment-related side effects, clinical trial participation, and practical or emotional support, which can help patients stay up to date on ICI advances. However, lack of Internet access and limitations in computer literacy can be potential barriers to accessing these resources. 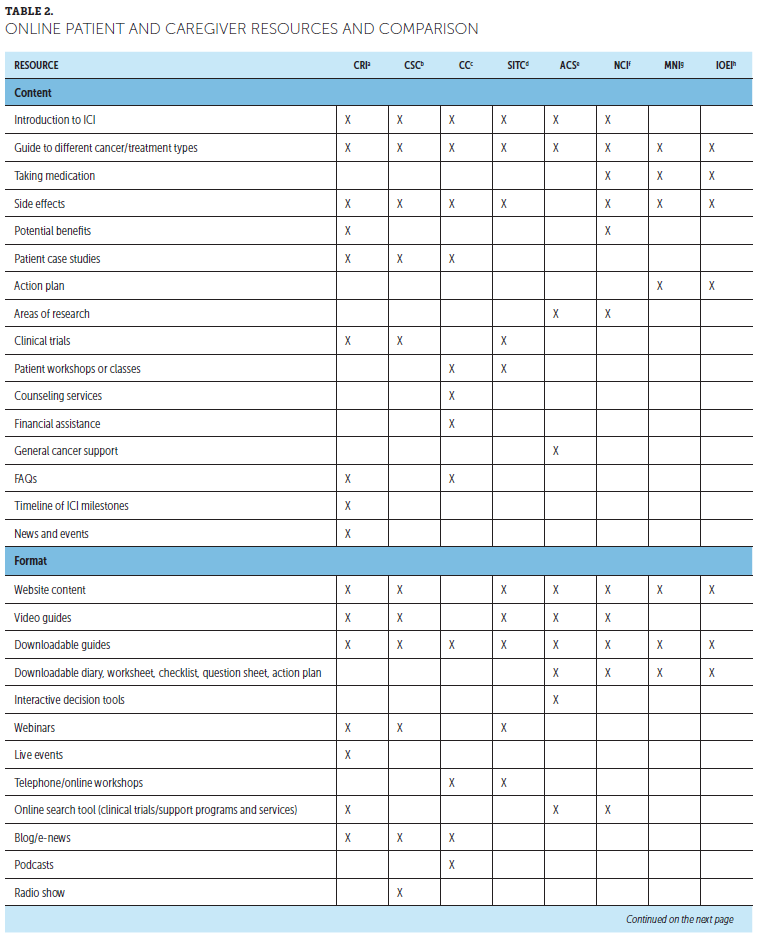
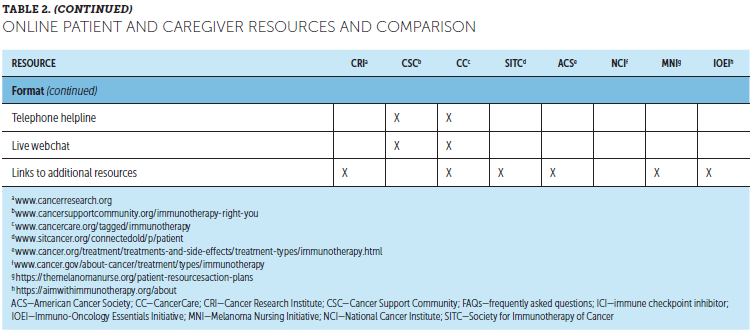
HCPs should be aware that not all online sources of information are reliable. In addition, availability of multiple patient resources can lead to contradictory information and be confusing for some patients (Nagler & LoRusso, 2017). A need for consistent patient education was identified through evaluation of existing educational processes at one U.S. cancer center (Hoff & Tonne, 2017). Survey responses from 154 interprofessional team members within inpatient and ambulatory care settings identified five key areas for improvement in consistency: individual learning needs assessment, approach to education delivery, resource standardization and availability, handoff processes between departments and disciplines, and documentation of learner progress. Addressing these inconsistencies reduced patient confusion and increased trust and satisfaction (Hoff & Tonne, 2017). In another U.S. cancer center, implementing a standardized approach to ICI education improved patient outcomes, including decreased serious AEs and emergency department visits, and improved ability to continue treatment related to early symptom recognition (Fradkin et al., 2017). Providing concise, consistent, and relevant patient education is critical for empowering patients and caregivers to manage side effects of ICIs (Fee-Schroeder et al., 2013).
Materials are available to support continuing patient education regarding ICIs, particularly potential side effects. Patient diaries, questionnaires, or standard assessment forms are recommended to help patients identify, keep track of, and report any potential side effects (Brahmer et al., 2018). Patient and caregiver guides are available in various formats, including frequently asked questions for discussion between patients and their cancer team. Wallet cards detailing symptoms to monitor and how to notify the cancer team may be an effective tool for empowering patients to recognize and manage potential irAEs (Brahmer et al., 2018; Fecher et al., 2013). When patients are away from their treating medical center or care teams, patients who carry wallet cards can help to facilitate communication between providers, particularly if emergency situations arise (Fecher et al., 2013; Oncology Nursing Society, 2019). The Oncology Nursing Society has developed an ICI wallet card for use by patients and their HCPs; copies of the card and additional information are available at www.ons.org/toolkits/immunotherapy-patient-wallet-card-1. 
Conclusion
ICIs represent a fast-developing cancer treatment option; patients with multiple types of malignancies can now potentially receive ICI therapy. The provision of timely, consistent, relevant, and personalized information for patients and caregivers is, therefore, essential. General learnings from patient education on chemotherapy suggest that educational resources should include a variety of teaching strategies that consider individual patient needs and preferences and are oriented toward patient empowerment. Although pretreatment patient education is essential, continued support and assessment of learning needs should also be made available. ICI-specific patient education should be developed with the input of an interprofessional team and regularly reviewed in line with emerging guidelines. Key messages for patients receiving ICIs include expected timing of therapeutic response and corresponding irAEs, importance of early irAE identification for effective management, and the unique ability of ICIs to influence immune response even after discontinuation. Several online resources are available to support patients and caregivers in understanding and managing ICIs. Additional patient supporting materials include symptom diaries or questionnaires and wallet cards to notify HCPs of ICI treatment. Oncology nurses play a vital role in ensuring that patients receive appropriate education related to their ICIs, which can support improved early irAE detection and appropriate intervention. 
About the Author(s)
Laura S. Wood, RN, MSN, OCN®, is a renal cancer research coordinator at the Cleveland Clinic Taussig Cancer Center in Ohio; Nancy P. Moldawer, RN, MSN, is a genitourinary research program coordinator at Cedars-Sinai Medical Center in Los Angeles, CA; and Colleen Lewis, MSN, ANP-BC, AOCNP®, is a lead nurse practitioner in the Winship Cancer Institute at Emory University in Atlanta, GA. The authors take full responsibility for this content. During the writing of this article, Wood was supported by funding from Bristol-Myers Squibb, Merck & Co., and Pfizer. Wood has previously consulted for Merck & Co. Moldawer has previously served on speakers bureaus for Exelixis. Lewis has previously consulted for Bristol-Myers Squibb. Medical writing support was provided by Katherine Baria, PhD, and editorial support was provided by Jay Rathi, MA, both of Spark Medica, Inc., through funding from Bristol-Myers Squibb. The article has been reviewed by independent peer reviewers to ensure that it is objective and free from bias. Wood can be reached at woodl@ccf.org, with copy to CJONEditor@ons.org. (Submitted November 2018. Accepted December 30, 2018.)
References
Abdel-Wahab, N., Alshawa, A., & Suarez-Almazor, M.E. (2017). Adverse events in cancer immunotherapy. Advances in Experimental Medicine and Biology, 995, 155–174. https://doi.org/10.1007/978-3-319-53156-4_8
Aim With Immunotherapy Foundation. (2019). Health care provider resources for optimizing IO therapy. Retrieved from https://aimwithimmunotherapy.org
Anadkat, M.J., & Cowen, E.W. (2018). Immunotherapy and skin side effects. JAMA Dermatology, 154, 744. https://doi.org/10.1001/jamadermatol.2018.0269
Aspiras, A., Power, N., & Gonzalo, M. (2018, January). Survey analysis: Assessing the needs of immunotherapy patients, caregivers, and health care providers. Paper presented at the ASCO-SITC Clinical Immuno-Oncology Symposium, San Francisco, CA.
Association of Community Cancer Centers. (2016). More information please: Online patient resources in immuno-oncology. Retrieved from https://accc-iclio.org/resources/more-information-please-online-patient…
AstraZeneca. (2018). ImfinziTM (durvalumab) [Package insert]. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761069s002lbl…
Australian Cancer Survivorship Centre. (2016). Needs assessment tools for post-treatment cancer survivors: Literature review. Retrieved from https://www.petermac.org/sites/default/files/page/downloads/Needs_Asses…
Bayer, V., Amaya, B., Baniewicz, D., Callahan, C., Marsh, L., & McCoy, A.S. (2017). Cancer immunotherapy: An evidence-based overview and implications for practice. Clinical Journal of Oncology Nursing, 21(2, Suppl.), 13–21. https://doi.org/10.1188/17.CJON.S2.13-21
Beatty, G.L., & Gladney, W.L. (2015). Immune escape mechanisms as a guide for cancer immunotherapy. Clinical Cancer Research, 21, 687–692. https://doi.org/10.1158/1078-0432.CCR-14-1860
Brahmer, J.R., Lacchetti, C., Schneider, B.J., Atkins, M.B., Brassil, K.J., Caterino, J.M., . . . Thompson, J.A. (2018). Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology, 36, 1714–1768. https://doi.org/10.1200/jco.2017.77.6385
Bristol-Myers Squibb. (2018a). Opdivo® (nivolumab) [Package insert]. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125554s058lbl…
Bristol-Myers Squibb. (2018b). Yervoy® (ipilimumab) [Package insert]. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125377s094lbl…
Canadian Partnership Against Cancer. (2018). Living with cancer: A report on the patient experience. Retrieved from http://www.virtualhospice.ca/Assets/CPAC_PatientExperienceReport_ENGLIS…
Cancer Support Community. (2018). Immunotherapy side effects. Retrieved from https://www.cancersupportcommunity.org/immunotherapy-side-effects
Cipolat Mis, C., Truccolo, I., Ravaioli, V., Cocchi, S., Gangeri, L., Mosconi, P., . . . De Paoli, P. (2015). Making patient centered care a reality: A survey of patient educational programs in Italian Cancer Research and Care Institutes. BMC Health Services Research, 15, 298. https://doi.org/10.1186/s12913-015-0962-5
de Mello, R.A., Veloso, A.F., Esrom Catarina, P., Nadine, S., & Antoniou, G. (2016). Potential role of immunotherapy in advanced non-small-cell lung cancer. OncoTargets and Therapy, 10, 21–30. https://doi.org/10.2147/OTT.S90459
Dine, J., Gordon, R., Shames, Y., Kasler, M.K., & BartonBurke, M. (2017). Immune checkpoint inhibitors: An innovation in immunotherapy for the treatment and management of patients with cancer. Asia-Pacific Journal of Oncology Nursing, 4, 127–135. https://doi.org/10.4103/apjon.apjon_4_17
Dougan, M., & Dranoff, G. (2009). The immune response to tumors. Current Protocols in Immunology, 85, 20.11.1–20.11.4. https://doi.org/10.1002/0471142735.im2011s85.85
Fay, A.P., Moreira, R.B., Nunes Filho, P.R.S., Albuquerque, C., & Barrios, C.H. (2016). The management of immune-related adverse events associated with immune checkpoint blockade. Expert Review of Quality of Life in Cancer Care, 1, 89–97. https://doi.org/10.1080/23809000.2016.1142827
Fecher, L.A., Agarwala, S.S., Hodi, F.S., & Weber, J.S. (2013). Ipilimumab and its toxicities: A multidisciplinary approach. Oncologist, 18, 733–743. https://doi.org/10.1634/theoncologist.2012-0483
Fee-Schroeder, K., Howell, L., Kokal, J., Bjornsen, S., Christensen, S., Hathaway, J., . . . Vickers, K.S. (2013). Empowering individuals to self-manage chemotherapy side effects. Clinical Journal of Oncology Nursing, 17, 369–371. https://doi.org/10.1188/13.CJON.369-371
Fradkin, M., Barbarotta, L., Duffield, E., Guttman, K., Randall-Doran, M., & Tuttle, L. (2017, May). Leveraging technology to optimize the care of patients treated with immunotherapy [Abstract 31]. Paper presented at the Oncology Nursing Society Annual Congress, Denver, CO.
Garcia, S. (2014). The effects of education on anxiety levels in patients receiving chemotherapy for the first time: An integrative review. Clinical Journal of Oncology Nursing, 18, 516–521. https://doi.org/10.1188/14.CJON.18-05AP
Genentech. (2018). Tecentriq® (atezolizumab) [Package insert]. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761034s005lbl…
George, G.C., Buford, A., Hess, K., Piha-Paul, S.A., Zinner, R., Subbiah, V., . . . Hong, D.S. (2018). Cancer-related internet use and online social networking among patients in an early-phase clinical trials clinic at a comprehensive cancer center. JCO Clinical Cancer Informatics, 2, 1–14. https://doi.org/10.1200/CCI.17.00030
Goldsmith, J.V., & Terui, S. (2018). Family oncology caregivers and relational health literacy. Challenges, 9, 35. https://doi.org/10.3390/challe9020035
Haanen, J.B., Thienen, H.V., & Blank, C.U. (2015). Toxicity patterns with immunomodulating antibodies and their combinations. Seminars in Oncology, 42, 423–428. https://doi.org/10.1053/j.seminoncol.2015.02.011
Haanen, J.B.A.G., Carbonnel, F., Robert, C., Kerr, K.M., Peters, S., Larkin, J., & Jordan, K. (2017). Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 28(Suppl. 4), iv119–iv142. https://doi.org/10.1093/annonc/mdx225
Hoff, B., & Tonne, H. (2017). Providing consistent patient education across the continuum of cancer care [Abstract 187]. Journal of Clinical Oncology, 35(Suppl. 8), 187.
Jewitt, N., Hope, A.J., Milne, R., Le, L.W., Papadakos, J., Abdelmutti, N., . . . Giuliani, M.E. (2016). Development and evaluation of patient education materials for elderly lung cancer patients. Journal of Cancer Education, 31, 70–74. https://doi.org/10.1007/s13187-014-0780-1
Kannan, R., Madden, K., & Andrews, S. (2014). Primer on immuno-oncology and immune response. Clinical Journal of Oncology Nursing, 18, 311–317. https://doi.org/10.1188/14.CJON.311-317
Kottschade, L., Brys, A., Peikert, T., Ryder, M., Raffals, L., Brewer, J., . . . Markovic, S. (2016). A multidisciplinary approach to toxicity management of modern immune checkpoint inhibitors in cancer therapy. Melanoma Research, 26, 469–480.
Kwon, E.D., Drake, C.G., Scher, H.I., Fizazi, K., Bossi, A., van den Eertwegh, A.J., . . . Gerritsen, W.R. (2014). Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncology, 15, 700–712. https://doi.org/10.1016/S1470-2045(14)70189-5
Lewis, C. (2016). Programmed death-1 inhibition in cancer with a focus on non-small cell lung cancer: Rationale, nursing implications, and patient management strategies. Clinical Journal of Oncology Nursing, 20, 319–326. https://doi.org/10.1188/16.CJON.319-326
Marcus, C. (2014). Strategies for improving the quality of verbal patient and family education: A review of the literature and creation of the EDUCATE model. Health Psychology and Behavioral Medicine, 2, 482–495. https://doi.org/10.1080/21642850.2014.900450
Mekuria, A.B., Erku, D.A., & Belachew, S.A. (2016). Preferred information sources and needs of cancer patients on disease symptoms and management: A cross-sectional study. Patient Preference and Adherence, 10, 1991–1997. https://doi.org/10.2147/PPA.S116463
Melanoma Nursing Initiative. (2017). Patient resources/action plans. Retrieved from http://themelanomanurse.org/patient-resourcesaction-plans
Merck & Co. (2018). Keytruda® (pembrolizumab) [Package insert]. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125514s034lbl…
Michot, J.M., Bigenwald, C., Champiat, S., Collins, M., Carbonnel, F., Postel-Vinay, S., . . . Lambotte, O. (2016). Immune-related adverse events with immune checkpoint blockade: A comprehensive review. European Journal of Cancer, 54, 139–148.
Nagler, R.H., & LoRusso, S.M. (2017). Conflicting information and message competition in health and risk messaging. Oxford Research Encyclopedias. https://doi.org/10.1093/acrefore/9780190228613.013.292
National Cancer Institute. (2018). Immunotherapy to treat cancer. Retrieved from https://www.cancer.gov/about-cancer/treatment/types/immunotherapy
Oncology Nursing Society. (2019). Immunotherapy wallet cards. Retrieved from https://www.ons.org/toolkits/immunotherapy-patient-wallet-card-1
Pfizer, Inc. (2017). Bavencio® (avelumab) [Package insert]. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761049s000lbl…
Portz, D., & Johnston, M.P. (2014). Implementation of an evidence-based education practice change for patients with cancer. Clinical Journal of Oncology Nursing, 18(Suppl.), 36–40. https://doi.org/10.1188/14.CJON.S2.36-40
Postow, M.A., Callahan, M.K., & Wolchok, J.D. (2015). Immune checkpoint blockade in cancer therapy. Journal of Clinical Oncology, 33, 1974–1982. https://doi.org/10.1200/JCO.2014.59.4358
Puzanov, I., Diab, A., Abdallah, K., Bingham, C.O., 3rd, Brogdon, C., Dadu, R., . . . Ernstoff, M.S. (2017). Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. Journal for Immunotherapy of Cancer, 5, 95. https://doi.org/10.1186/s40425-017-0300-z
Schadendorf, D., Wolchok, J.D., Hodi, F.S., Chiarion-Sileni, V., Gonzalez, R., Rutkowski, P., . . . Postow, M.A. (2017). Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: A pooled analysis of randomized phase II and III trials. Journal of Clinical Oncology, 35, 3807–3814. https://doi.org/10.1200/JCO.2017.73.2289
Shahsavari, H., Matory, P., Zare, Z., Taleghani, F., & Kaji, M.A. (2015). Effect of self-care education on the quality of life in patients with breast cancer. Journal of Education and Health Promotion, 4, 70. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4944611/
Shea-Budgell, M.A., Kostaras, X., Myhill, K.P., & Hagen, N.A. (2014). Information needs and sources of information for patients during cancer follow-up. Current Oncology, 21, 165–173. https://doi.org/10.3747/co.21.1932
Society for Immunotherapy of Cancer. (2017). SITC connectED patient resources. Retrieved from https://www.sitcancer.org/connected/p/patient
Spain, L., Diem, S., & Larkin, J. (2016). Management of toxicities of immune checkpoint inhibitors. Cancer Treatment Reviews, 44, 51–60. https://doi.org/10.1016/j.ctrv.2016.02.001
Tian, J., Lia, L.N., & Cheng, G.C. (2015). Relationships between patient knowledge and the severity of side effects, daily nutrient intake, psychological status, and performance status in lung cancer patients. Current Oncology, 22(4), e254–e258. https://doi.org/10.3747/co.22.2366
U.S. Food and Drug Administration. (2018). FDA grants nivolumab accelerated approval for third-line treatment of metastatic small cell lung cancer. Retrieved from https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm617370.htm
Valenti, R.B. (2014). Chemotherapy education for patients with cancer: A literature review. Clinical Journal of Oncology Nursing, 18, 637–640. https://doi.org/10.1188/14.CJON.637-640
Weber, J.S., Postow, M., Lao, C.D., & Schadendorf, D. (2016). Management of adverse events following treatment with anti-programmed death-1 agents. The Oncologist, 21, 1230–1240. https://doi.org/10.1634/theoncologist.2016-0055
Wolchok, J.D., Hoos, A., O’Day, S., Weber, J.S., Hamid, O., Lebbé, C., . . . Hodi, S. (2009). Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clinical Cancer Research 15, 7412–7420. https://doi.org/10.1158/1078-0432.CCR-09-1624
Zimmer, L., Goldinger, S.M., Hofmann, L., Loquai, C., Ugurel, S., Thomas, I., . . . Heinzerling, L.M. (2016). Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. European Journal of Cancer, 60, 210–225. https://doi.org/10.1016/j.ejca.2016.02.024




